Operando X-Ray Absorption Spectroscopy Shows Iron Oxidation Is Concurrent with Oxygen Evolution in Cobalt–Iron (Oxy)hydroxide Electrocatalysts
Correction(s) for this article
-
Corrigendum: Operando X-Ray Absorption Spectroscopy Shows Iron Oxidation Is Concurrent with Oxygen Evolution in Cobalt–Iron (Oxy)hydroxide Electrocatalysts
- Lisa J. Enman,
- Michaela Burke Stevens,
- Meir Haim Dahan,
- Michael R. Nellist,
- Maytal Caspary Toroker,
- Shannon W. Boettcher,
- Volume 58Issue 7Angewandte Chemie International Edition
- pages: 1867-1867
- First Published online: November 14, 2018
Dr. Lisa J. Enman
Department of Chemistry & Biochemistry and the Materials Science Institute, University of Oregon, Eugene, OR, 97403 USA
Search for more papers by this authorDr. Michaela Burke Stevens
Department of Chemistry & Biochemistry and the Materials Science Institute, University of Oregon, Eugene, OR, 97403 USA
Search for more papers by this authorMeir Haim Dahan
Department of Materials Science & Engineering and The Nancy & Stephen Grand Technion Energy Program, Technion—Israel Institute of Technology, Haifa, 3200003 Israel
Search for more papers by this authorDr. Michael R. Nellist
Department of Chemistry & Biochemistry and the Materials Science Institute, University of Oregon, Eugene, OR, 97403 USA
Search for more papers by this authorCorresponding Author
Prof. Maytal Caspary Toroker
Department of Materials Science & Engineering and The Nancy & Stephen Grand Technion Energy Program, Technion—Israel Institute of Technology, Haifa, 3200003 Israel
Search for more papers by this authorCorresponding Author
Prof. Shannon W. Boettcher
Department of Chemistry & Biochemistry and the Materials Science Institute, University of Oregon, Eugene, OR, 97403 USA
Search for more papers by this authorDr. Lisa J. Enman
Department of Chemistry & Biochemistry and the Materials Science Institute, University of Oregon, Eugene, OR, 97403 USA
Search for more papers by this authorDr. Michaela Burke Stevens
Department of Chemistry & Biochemistry and the Materials Science Institute, University of Oregon, Eugene, OR, 97403 USA
Search for more papers by this authorMeir Haim Dahan
Department of Materials Science & Engineering and The Nancy & Stephen Grand Technion Energy Program, Technion—Israel Institute of Technology, Haifa, 3200003 Israel
Search for more papers by this authorDr. Michael R. Nellist
Department of Chemistry & Biochemistry and the Materials Science Institute, University of Oregon, Eugene, OR, 97403 USA
Search for more papers by this authorCorresponding Author
Prof. Maytal Caspary Toroker
Department of Materials Science & Engineering and The Nancy & Stephen Grand Technion Energy Program, Technion—Israel Institute of Technology, Haifa, 3200003 Israel
Search for more papers by this authorCorresponding Author
Prof. Shannon W. Boettcher
Department of Chemistry & Biochemistry and the Materials Science Institute, University of Oregon, Eugene, OR, 97403 USA
Search for more papers by this authorGraphical Abstract
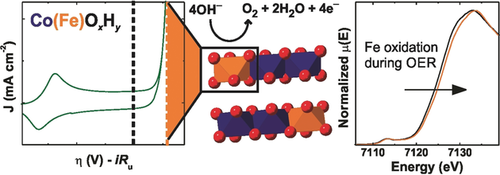
Fe-containing Co and Ni (oxy)hydroxides are the fastest-known catalysts for water oxidation in alkaline conditions. Fe is essential to the high activity, but its role in the mechanism is under active investigation. Now it is shown that oxygen evolution is concurrent with the oxidation of Fe cations and shortening of Fe−O bonds. The results are consistent with density functional theory calculations and may indicate Fe-based active sites.
Abstract
Iron cations are essential for the high activity of nickel and cobalt-based (oxy)hydroxides for the oxygen evolution reaction, but the role of iron in the catalytic mechanism remains under active investigation. Operando X-ray absorption spectroscopy and density functional theory calculations are used to demonstrate partial Fe oxidation and a shortening of the Fe−O bond length during oxygen evolution on Co(Fe)OxHy. Cobalt oxidation during oxygen evolution is only observed in the absence of iron. These results demonstrate a different mechanism for water oxidation in the presence and absence of iron and support the hypothesis that oxidized iron species are involved in water-oxidation catalysis on Co(Fe)OxHy.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201808818-sup-0001-misc_information.pdf1.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1L. Trotochaud, J. K. Ranney, K. N. Williams, S. W. Boettcher, J. Am. Chem. Soc. 2012, 134, 17253–17261.
- 2M. S. Burke, S. Zou, L. J. Enman, J. E. Kellon, C. A. Gabor, E. Pledger, S. W. Boettcher, J. Phys. Chem. Lett. 2015, 6, 3737–3742.
- 3D. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M.-J. Cheng, D. Sokaras, T.-C. Weng, R. Alonso-Mori, et al., J. Am. Chem. Soc. 2015, 137, 1305–1313.
- 4M. Görlin, J. F. De Araujo, H. Schmies, D. Bernsmeier, S. Dresp, M. Gliech, Z. Jusys, P. Chernev, R. Kraehnert, H. Dau, et al., J. Am. Chem. Soc. 2017, 139, 2070–2082.
- 5M. Görlin, P. Chernev, J. Ferreira de Araújo, T. Reier, S. Dresp, B. Paul, R. Krähnert, H. Dau, P. Strasser, J. Am. Chem. Soc. 2016, 138, 5603–5614.
- 6R. D. L. Smith, C. Pasquini, S. Loos, P. Chernev, K. Klingan, P. Kubella, M. R. Mohammadi, D. Gonzalez-Flores, H. Dau, Nat. Commun. 2017, 8, 2022.
- 7M. K. Bates, Q. Jia, H. Doan, W. Liang, S. Mukerjee, ACS Catal. 2016, 6, 155–161.
- 8L. Gong, X. Y. E. Chng, Y. Du, S. Xi, B. S. Yeo, ACS Catal. 2018, 8, 807–814.
- 9N. Li, D. K. Bediako, R. G. Hadt, D. Hayes, T. J. Kempa, F. von Cube, D. C. Bell, L. X. Chen, D. G. Nocera, Proc. Natl. Acad. Sci. USA 2017, 114, 1486–1491.
- 10J. Y. C. Chen, L. Dang, H. Liang, W. Bi, J. B. Gerken, S. Jin, E. E. Alp, S. S. Stahl, J. Am. Chem. Soc. 2015, 137, 15090–15093.
- 11B. M. Hunter, N. B. Thompson, A. M. Müller, G. R. Rossman, M. G. Hill, J. R. Winkler, H. B. Gray, Joule 2018, 2, 747–763.
- 12V. Fidelsky, M. C. Toroker, Phys. Chem. Chem. Phys. 2017, 19, 7491–7497.
- 13Z. K. Goldsmith, A. K. Harshan, J. B. Gerken, M. Vörös, G. Galli, S. S. Stahl, S. Hammes-Schiffer, Proc. Natl. Acad. Sci. USA 2017, 114, 3050–3055.
- 14B. Hunter, J. Winkler, H. Gray, Molecules 2018, 23, 903.
- 15M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith, S. W. Boettcher, J. Am. Chem. Soc. 2015, 137, 3638–3648.
- 16L. Trotochaud, S. L. Young, J. K. Ranney, S. W. Boettcher, J. Am. Chem. Soc. 2014, 136, 6744–6753.
- 17B. M. Hunter, W. Hieringer, J. Winkler, H. B. Gray, A. M. Müller, Energy Environ. Sci. 2016, 23, 1–22.
- 18M. B. Stevens, C. D. M. Trang, L. J. Enman, J. Deng, S. W. Boettcher, J. Am. Chem. Soc. 2017, 139, 11361–11364.
- 19H. Yang, Y. Liu, S. Luo, Z. Zhao, X. Wang, Y. Luo, Z. Wang, J. Jin, J. Ma, ACS Catal. 2017, 7, 5557–5567.
- 20F. Song, X. Hu, Nat. Commun. 2014, 5, 4477.
- 21J. Fester, M. García-Melchor, A. S. Walton, M. Bajdich, Z. Li, L. Lammich, A. Vojvodic, J. V. Lauritsen, L. R. Merte, M. A. Henderson, et al., Nat. Commun. 2017, 8, 14169.
- 22M. Favaro, J. Yang, S. Nappini, E. Magnano, F. M. Toma, E. J. Crumlin, J. Yano, I. D. Sharp, J. Am. Chem. Soc. 2017, 139, 8960–8970.
- 23E. Fabbri, M. Nachtegaal, T. Binninger, X. Cheng, B. J. Kim, J. Durst, F. Bozza, T. Graule, R. Schäublin, L. Wiles, et al., Nat. Mater. 2017, 16, 925–931.
- 24L. Calvillo, F. Carraro, O. Vozniuk, V. Celorrio, L. Nodari, A. E. Russell, D. Debellis, D. Fermin, F. Cavani, S. Agnoli, et al., J. Mater. Chem. A 2018, 6, 7034–7041.
- 25D. González-Flores, K. Klingan, P. Chernev, S. Loos, M. R. Mohammadi, C. Pasquini, P. Kubella, I. Zaharieva, R. D. L. Smith, H. Dau, Sustain. Energy Fuels 2018, 2, 1986–1994.
- 26J. G. McAlpin, Y. Surendranath, M. Dincǎ, T. A. Stich, S. A. Stoian, W. H. Casey, D. G. Nocera, R. D. Britt, J. Am. Chem. Soc. 2010, 132, 6882–6883.
- 27R. G. Hadt, D. Hayes, C. N. Brodsky, A. M. Ullman, D. M. Casa, M. H. Upton, D. G. Nocera, L. X. Chen, J. Am. Chem. Soc. 2016, 138, 11017–11030.
- 28D. Friebel, M. Bajdich, B. S. Yeo, M. W. Louie, D. J. Miller, H. Sanchez Casalongue, F. Mbuga, T. C. Weng, D. Nordlund, D. Sokaras, et al., Phys. Chem. Chem. Phys. 2013, 15, 17460–17467.
- 29M. Zhang, M. De Respinis, H. Frei, Nat. Chem. 2014, 6, 362–367.
- 30A. Deb, J. Ralph, E. Cairns, U. Bergmann, Phys. Rev. B 2006, 73, 1–9.
- 31J. Blasco, B. Aznar, J. García, G. Subías, J. Herrero-Martín, J. Stankiewicz, Phys. Rev. B Condens. Matter Mater. Phys. 2008, 77, 1–10.
- 32M. Balasubramanian, C. A. Melendres, S. Mini, J. Phys. Chem. B 2000, 104, 4300–4306.
- 33S. P. Cramer, T. K. Eccles, F. W. Kutzler, K. O. Hodgson, L. E. Mortenson, J. Am. Chem. Soc. 1976, 98, 1287–1288.
- 34R. L. Blake, R. E. Hessevick, T. Zoltai, L. W. Finger, Am. Mineral. 1966, 51, 123–129.
- 35H. Christensen, A. N. Christensen, U. Turpeinen, A. F. Andresen, O. Smidsrød, C.-O. Pontchour, P. Phavanantha, S. Pramatus, B. N. Cyvin, S. J. Cyvin, Acta Chem. Scand. Ser. A 1978, 32, 87–88.
- 36G. Kresse, J. Hafner, Phys. Rev. B 1993, 47, 558–561.
- 37G. Kresse, J. Furthmüller, Comput. Mater. Sci. 1996, 6, 15–50.
- 38S. Dudarev, G. Botton, Phys. Rev. B 1998, 57, 1505–1509.
- 39I. C. Man, H.-Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov, J. Rossmeisl, ChemCatChem 2011, 3, 1159–1165.
- 40K. Momma, F. Izumi, J. Appl. Crystallogr. 2011, 44, 1272–1276.
- 41Y. Surendranath, M. W. Kanan, D. G. Nocera, J. Am. Chem. Soc. 2010, 132, 16501–16509.
- 42C. Costentin, D. G. Nocera, Proc. Natl. Acad. Sci. USA 2017, 114, 13380–13384.