Tuning Intramolecular Förster Resonance Energy Transfer and Activating Intramolecular Singlet Fission
Giulia Lavarda
Departamento de Química Orgánica and Institute for Advanced Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
These authors contributed equally to this work.
Search for more papers by this authorDr. Johannes Zirzlmeier
Department of Chemistry and Pharmacy & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstrasse 3, 91058 Erlangen, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Marco Gruber
Department of Chemistry and Pharmacy & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Nikolaus-Fiebiger-Strasse 10, 91058 Erlangen, Germany
Search for more papers by this authorParisa R. Rami
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorCorresponding Author
Prof. Rik R. Tykwinski
Department of Chemistry and Pharmacy & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Nikolaus-Fiebiger-Strasse 10, 91058 Erlangen, Germany
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Tomás Torres
Departamento de Química Orgánica and Institute for Advanced Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
IMDEA-Nanociencia, Campus de Cantoblanco, 28049 Madrid, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Dirk M. Guldi
Department of Chemistry and Pharmacy & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstrasse 3, 91058 Erlangen, Germany
Search for more papers by this authorGiulia Lavarda
Departamento de Química Orgánica and Institute for Advanced Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
These authors contributed equally to this work.
Search for more papers by this authorDr. Johannes Zirzlmeier
Department of Chemistry and Pharmacy & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstrasse 3, 91058 Erlangen, Germany
These authors contributed equally to this work.
Search for more papers by this authorDr. Marco Gruber
Department of Chemistry and Pharmacy & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Nikolaus-Fiebiger-Strasse 10, 91058 Erlangen, Germany
Search for more papers by this authorParisa R. Rami
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorCorresponding Author
Prof. Rik R. Tykwinski
Department of Chemistry and Pharmacy & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Nikolaus-Fiebiger-Strasse 10, 91058 Erlangen, Germany
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Tomás Torres
Departamento de Química Orgánica and Institute for Advanced Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049 Madrid, Spain
IMDEA-Nanociencia, Campus de Cantoblanco, 28049 Madrid, Spain
Search for more papers by this authorCorresponding Author
Prof. Dr. Dirk M. Guldi
Department of Chemistry and Pharmacy & Interdisciplinary Center for Molecular Materials (ICMM), Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Egerlandstrasse 3, 91058 Erlangen, Germany
Search for more papers by this authorDedicated to Professor Dan Meyerstein on the occasion of his 80th birthday
Graphical Abstract
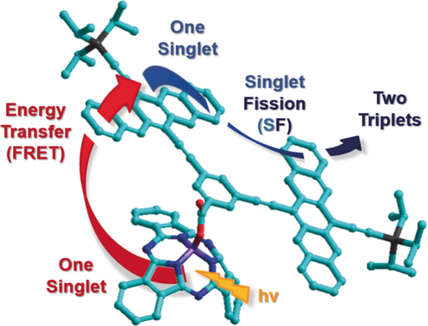
FRETter by design: Three subphthalocyanine (SubPc)/pentacene dimer (Pnc2) conjugates were prepared for the synergy of panchromatic light absorption, energy transfer, and intramolecular singlet fission (SF). Transient absorption measurements confirmed the reaction sequence of light harvesting by the SubPcs, unidirectional Förster resonance energy transfer (FRET) from the SubPcs to the Pnc2, and almost quantitative intramolecular SF.
Abstract
The synergy of panchromatic absorption throughout most of the visible range of the solar spectrum and intramolecular singlet fission (SF) has been realized in a series of conjugates featuring different light-harvesting subphthalocyanines (SubPcs) and an energy accepting pentacene dimer (Pnc2). At the focal point was a modular SubPc approach, which was based on decorating the SubPc core with different peripheral substituents to tailor and fine-tune their optical properties. Transient absorption measurements assisted in corroborating that the SubPcs act as energy-transfer antennas by means of unidirectional and quantitative intramolecular Förster resonance energy transfer (FRET) to the Pnc2, where an intramolecular SF affords triplet quantum yields reaching unity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201808652-sup-0001-misc_information.pdf3.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. C. Beard, J. C. Johnson, J. M. Luther, A. J. Nozik, Philos. Trans. R. Soc. London Ser. A 2015, 373, 20140412.
- 2
- 2aM. B. Smith, J. Michl, Chem. Rev. 2010, 110, 6891–6936;
- 2bM. B. Smith, J. Michl, Annu. Rev. Phys. Chem. 2013, 64, 361–386.
- 3E. G. Fuemmeler, S. N. Sanders, A. B. Pun, E. Kumarasamy, T. Zeng, K. Miyata, M. L. Steigerwald, X. Y. Zhu, M. Y. Sfeir, L. M. Campos, N. Ananth, ACS Cent. Sci. 2016, 2, 316–324.
- 4
- 4aW. L. Chan, M. Ligges, A. Jailaubekov, L. Kaake, L. Miaja-Avila, X. Y. Zhu, Science 2011, 334, 1541–1545;
- 4bW. L. Chan, M. Ligges, X. Y. Zhu, Nat. Chem. 2012, 4, 840–845;
- 4cR. W. A. Havenith, H. D. de Gier, R. Broer, Mol. Phys. 2012, 110, 2445–2454;
- 4dW. L. Chan, T. C. Berkelbach, M. R. Provorse, N. R. Monahan, J. R. Tritsch, M. S. Hybertsen, D. R. Reichman, J. Gao, X. Y. Zhu, Acc. Chem. Res. 2013, 46, 1321–1329;
- 4eX. Feng, A. V. Luzanov, A. I. Krylov, J. Phys. Chem. Lett. 2013, 4, 3845–3852;
- 4fD. Beljonne, H. Yamagata, J. L. Brédas, F. C. Spano, Y. Olivier, Phys. Rev. Lett. 2013, 110, 226402;
- 4gT. C. Berkelbach, M. S. Hybertsen, D. R. Reichman, J. Chem. Phys. 2013, 138, 114102;
- 4hT. C. Berkelbach, M. S. Hybertsen, D. R. Reichman, J. Chem. Phys. 2013, 138, 114103;
- 4iT. C. Berkelbach, M. S. Hybertsen, D. R. Reichman, J. Chem. Phys. 2014, 141, 074705;
- 4jT. Zeng, R. Hoffmann, N. Ananth, J. Am. Chem. Soc. 2014, 136, 5755–5764;
- 4kS. R. Yost, J. Lee, M. W. B. Wilson, T. Wu, D. P. McMahon, R. R. Parkhurst, N. J. Thompson, D. N. Congreve, A. Rao, K. Johnson, M. Y. Sfeir, M. G. Bawendi, T. M. Swager, R. H. Friend, M. A. Baldo, T. Van Voorhis, Nat. Chem. 2014, 6, 492–497;
- 4lN. Monahan, X.-Y. Zhu, Annu. Rev. Phys. Chem. 2015, 66, 601–618.
- 5
- 5aA. F. Schwerin, J. C. Johnson, M. B. Smith, P. Sreearunothai, D. Popović, J. Černý, Z. Havlas, I. Paci, A. Akdag, M. K. MacLeod, X. Chen, D. E. David, M. A. Ratner, J. R. Miller, A. J. Nozik, J. Michl, J. Phys. Chem. A 2010, 114, 1457–1473;
- 5bJ. C. Johnson, A. Akdag, M. Zamadar, X. Chen, A. F. Schwerin, I. Paci, M. B. Smith, Z. Havlas, J. R. Miller, M. A. Ratner, A. J. Nozik, J. Michl, J. Phys. Chem. B 2013, 117, 4680–4695;
- 5cJ. C. Johnson, J. Michl, Top. Curr. Chem. 2017, 375, 80.
- 6
- 6aS. W. Eaton, L. E. Shoer, S. D. Karlen, S. M. Dyar, E. A. Margulies, B. S. Veldkamp, C. Ramanan, D. A. Hartzler, S. Savikhin, T. J. Marks, M. R. Wasielewski, J. Am. Chem. Soc. 2013, 135, 14701–14712;
- 6bS. W. Eaton, S. A. Miller, E. A. Margulies, L. E. Shoer, R. D. Schaller, M. R. Wasielewski, J. Phys. Chem. A 2015, 119, 4151–4161;
- 6cE. A. Margulies, C. E. Miller, Y. Wu, L. Ma, G. C. Schatz, R. M. Young, M. R. Wasielewski, Nat. Chem. 2016, 8, 1120–1125.
- 7
- 7aJ. Zirzlmeier, D. Lehnherr, P. B. Coto, E. T. Chernick, R. Casillas, B. S. Basel, M. Thoss, R. R. Tykwinski, D. M. Guldi, Proc. Natl. Acad. Sci. USA 2015, 112, 5325–5330;
- 7bS. N. Sanders, E. Kumarasamy, A. B. Pun, M. T. Trinh, B. Choi, J. Xia, E. J. Taffet, J. Z. Low, J. R. Miller, X. Roy, X. Y. Zhu, M. L. Steigerwald, M. Y. Sfeir, L. M. Campos, J. Am. Chem. Soc. 2015, 137, 8965–8972;
- 7cS. Lukman, A. J. Musser, K. Chen, S. Athanasopoulos, C. K. Yong, Z. Zeng, Q. Ye, C. Chi, J. M. Hodgkiss, J. Wu, R. H. Friend, N. C. Greenham, Adv. Funct. Mater. 2015, 25, 5452–5461;
- 7dS. Lukman, K. Chen, J. M. Hodgkiss, D. H. P. Turban, N. D. M. Hine, S. Dong, J. Wu, N. C. Greenham, A. J. Musser, Nat. Commun. 2016, 7, 13622;
- 7eJ. Zirzlmeier, R. Casillas, S. R. Reddy, P. B. Coto, D. Lehnherr, E. T. Chernick, I. Papadopoulos, M. Thoss, R. R. Tykwinski, D. M. Guldi, Nanoscale 2016, 8, 10113–10123;
- 7fT. Sakuma, H. Sakai, Y. Araki, T. Mori, T. Wada, N. V. Tkachenko, T. Hasobe, J. Phys. Chem. A 2016, 120, 1867–1875;
- 7gM. J. Y. Tayebjee, S. N. Sanders, E. Kumarasamy, L. M. Campos, M. Y. Sfeir, D. R. McCamey, Nat. Phys. 2017, 13, 182–188;
- 7hE. Kumarasamy, S. N. Sanders, M. J. Y. Tayebjee, A. Asadpoordarvish, T. J. H. Hele, E. G. Fuemmeler, A. B. Pun, L. M. Yablon, J. Z. Low, D. W. Paley, J. C. Dean, B. Choi, G. D. Scholes, M. L. Steigerwald, N. Ananth, D. R. McCamey, M. Y. Sfeir, L. M. Campos, J. Am. Chem. Soc. 2017, 139, 12488–12494;
- 7iB. S. Basel, J. Zirzlmeier, C. Hetzer, B. T. Phelan, M. D. Krzyaniak, S. R. Reddy, P. B. Coto, N. E. Horwitz, R. M. Young, F. J. White, F. Hampel, T. Clark, M. Thoss, R. R. Tykwinski, M. R. Wasielewski, D. M. Guldi, Nat. Commun. 2017, 8, 15171.
- 8C. Hetzer, D. Guldi, R. R. Tykwinski, Chem. Eur. J. 2018, 24, 8245–8257.
- 9
- 9aB. Gobets, R. van Grondelle, Biochim. Biophys. Acta Bioenerg. 2001, 1507, 80–99;
- 9bR. Berera, R. van Grondelle, J. T. M. Kennis, Photosynth. Res. 2009, 101, 105–118.
- 10B. E. Hardin, J.-H. Yum, E. T. Hoke, Y. C. Jun, P. Péchy, T. Torres, M. L. Brongersma, M. K. Nazeeruddin, M. Grätzel, M. D. McGehee, Nano Lett. 2010, 10, 3077–3083.
- 11C. G. Claessens, D. González-Rodríguez, M. S. Rodríguez-Morgade, A. Medina, T. Torres, Chem. Rev. 2014, 114, 2192–2277.
- 12
- 12aM. E. El-Khouly, D. K. Ju, K.-Y. Kay, F. D′Souza, S. Fukuzumi, Chem. Eur. J. 2010, 16, 6193–6202;
- 12bJ.-Y. Liu, H.-S. Yeung, W. Xu, X. Li, D. K. P. Ng, Org. Lett. 2008, 10, 5421–5424;
- 12cK. A. Winterfeld, G. Lavarda, J. Guilleme, M. Sekita, D. M. Guldi, T. Torres, G. Bottari, J. Am. Chem. Soc. 2017, 139, 5520–5529;
- 12dA. V. Gorbunov, M. Garcia Iglesias, J. Guilleme, T. D. Cornelissen, W. S. C. Roelofs, T. Torres, D. González-Rodríguez, E. W. Meijer, M. Kemerink, Sci. Adv. 2017, 3, e 1701017;
- 12eC. Duan, G. Zango, M. García Iglesias, F. J. M. Colberts, M. M. Wienk, M. V. Martínez-Díaz, R. A. J. Janssen, T. Torres, Angew. Chem. Int. Ed. 2017, 56, 148–152; Angew. Chem. 2017, 129, 154–158;
- 12fM.-E. Ragoussi, T. Torres, Chem. Commun. 2015, 51, 3957–3972;
- 12gG. de la Torre, G. Bottari, T. Torres, Adv. Energy Mater. 2017, 7, 1601700.
- 13
- 13aP. D. Reusswig, D. N. Congreve, N. J. Thompson, M. A. Baldo, Appl. Phys. Lett. 2012, 101, 113304;
- 13bJ. Lee, P. Jadhav, P. D. Reusswig, S. R. Yost, N. J. Thompson, D. N. Congreve, E. Hontz, T. Van Voorhis, M. A. Baldo, Acc. Chem. Res. 2013, 46, 1300–1311.
- 14
- 14aC. G. Claessens, D. González-Rodríguez, B. del Rey, T. Torres, G. Mark, H.-P. Schuchmann, C. von Sonntag, J. G. MacDonald, R. S. Nohr, Eur. J. Org. Chem. 2003, 2547–2551;
- 14bD. González-Rodríguez, T. Torres, E. L. G. Denardin, D. Samios, V. Stefani, D. S. Corrêa, J. Organomet. Chem. 2009, 694, 1617–1622.
- 15J. Guilleme, D. González-Rodríguez, T. Torres, Angew. Chem. Int. Ed. 2011, 50, 3506–3509; Angew. Chem. 2011, 123, 3568–3571.
- 16A. Kunzmann, M. Gruber, R. Casillas, J. Zirzlmeier, M. Stanzel, W. Peukert, R. R. Tykwinski, D. M. Guldi, Angew. Chem. Int. Ed. 2018, 57, 10742–10747; Angew. Chem. 2018, 130, 10902–10907.
- 17See Supporting Information for details.
- 18A. Rodriguez-Serrano, V. Rai-Constapel, M. C. Daza, M. Doerr, C. M. Marian, Phys. Chem. Chem. Phys. 2015, 17, 11350–11358.
- 19Almost identical deactivation patterns were noted for 1 and 3, Supporting Information.
- 20R. D. Pensack, E. E. Ostroumov, A. J. Tilley, S. Mazza, C. Grieco, K. J. Thorley, J. B. Asbury, D. S. Seferos, J. E. Anthony, G. D. Scholes, J. Phys. Chem. Lett. 2016, 7, 2370–2375.
- 21J. Szczepanski, C. Wehlburg, M. Vala, Chem. Phys. Lett. 1995, 232, 221–228.
- 22Comparing the transient absorption features of 1(S1S0)/1(T1T1) with those of the intermediate reveals a 445 nm signature as the only appreciable difference. Note that the different triplet excited states in pentacene monomers and pentacene dimers, that is, a localized triplet excited state (T1), a correlated pair of triplet excited states of either singlet 1(T1T1), or quintet multiplicity 5(T1T1), and an uncorrelated pair of triplet excited states (T1 + T1), all feature nearly the exact same transient absorption features in the same solvent.
- 23B. S. Basel, J. Zirzlmeier, C. Hetzer, S. R. Reddy, B. T. Phelan, M. D. Krzyaniak, M. K. Volland, P. B. Coto, R. M. Young, T. Clark, M. Thoss, R. R. Tykwinski, M. R. Wasielewski, D. M. Guldi, Chem 2018, 4, 1092–1111.
- 24These values are in very good agreement with those found experimentally. Interestingly, a better spectral overlap between energy donor and acceptor in, for example, 6 does not lead to a larger rate constant when compared to 4 and 5. The fluorescence quantum yields of the SubPc energy donors, which are lowest for 3, overcompensate those benefits stemming from a larger spectral overlap integral.