The Diverse Reactivity of Disilenes Toward Isocyanides
Dedicated to Professor Robert West for his many inspiring contributions to silicon chemistry
Graphical Abstract
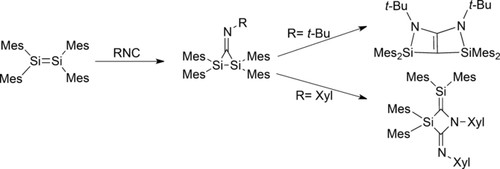
Twice the fun: In the addition of 2,6-dimethylphenyl isocyanide or tert-butyl isocyanide to tetramesityldisilene, the initially formed product is an iminodisilirane. These iminodisilirane intermediates react with a second equivalent of the isocyanide to give a 3-silaazetidine or a bicyclic double enamine, respectively.
Abstract
The addition of 2,6-dimethylphenyl isocyanide and t-butyl isocyanide to tetramesityldisilene was examined. In both cases, the initially formed product is an iminodisilirane; however, the iminodisiliranes are unstable under the reaction conditions and react with a second equivalent of the isocyanide to give either a 3-silaazetidine or a novel bicyclic double enamine, respectively. Taken together with the previous examples in the literature, the results demonstrate that subtle differences in the steric bulk of the disilene or the electronic effects of the isocyanide can lead to dramatic differences in the reaction pathway.