Access to P- and Axially Chiral Biaryl Phosphine Oxides by Enantioselective CpxIrIII-Catalyzed C−H Arylations
Yun-Suk Jang
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-, 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Łukasz Woźniak
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-, 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Julia Pedroni
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-, 1015 Lausanne, Switzerland
Search for more papers by this authorCorresponding Author
Prof. Dr. Nicolai Cramer
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-, 1015 Lausanne, Switzerland
Search for more papers by this authorYun-Suk Jang
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-, 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Łukasz Woźniak
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-, 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Julia Pedroni
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-, 1015 Lausanne, Switzerland
Search for more papers by this authorCorresponding Author
Prof. Dr. Nicolai Cramer
Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC LCSA, BCH 4305, CH-, 1015 Lausanne, Switzerland
Search for more papers by this authorGraphical Abstract
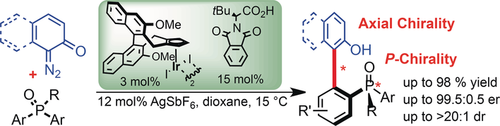
An enantioselective C−H arylation of phosphine oxides with o-quinone diazides is catalyzed by a CpxIrIII complex and chiral carboxylic acid co-catalyst. It provides a unifying access to P-chiral phosphine oxides, atropo-enantioselective construction of sterically demanding biaryl backbones, and a selective assembly of axial and P-chiral compounds in excellent yields and diastereo- and enantioselectivities.
Abstract
An enantioselective C−H arylation of phosphine oxides with o-quinone diazides catalyzed by an iridium(III) complex bearing an atropchiral cyclopentadienyl (Cpx) ligand and phthaloyl tert-leucine as co-catalyst is reported. The method allows access to a) P-chiral biaryl phosphine oxides, b) atropo-enantioselective construction of sterically demanding biaryl backbones, and also c) selective assembly of axial and P-chiral compounds in excellent yields and diastereo- and enantioselectivities. Enantiospecific reductions provide monodentate chiral phosphorus(III) compounds having structures and biaryl backbones with proven importance as ligands in asymmetric catalysis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201807749-sup-0001-misc_information.pdf17.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Privileged Chiral Ligands and Catalysts (Eds.: ), Wiley-VCH, Weinheim, 2011;
- 1bJ. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Sausalito, CA, 2010.
- 2 Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis (Eds.: ), Wiley, Hoboken, 2012.
- 3
- 3aF. Lagasse, H. B. Kagan, Chem. Pharm. Bull. 2000, 48, 315;
- 3b Phosphorus Ligands in Asymmetric Catalysis: Synthesis and Applications, Vol. 1–3 (Ed.: ), Wiley-VCH, Weinheim, 2008;
- 3cJ. Pedroni, N. Cramer, Chem. Commun. 2015, 51, 17647;
- 3dW. Fu, W. Tang, ACS Catal. 2016, 6, 4814.
- 4R. Noyori, Acc. Chem. Res. 1990, 23, 345.
- 5T. Hayashi, Acc. Chem. Res. 2000, 33, 354.
- 6J. Yin, S. L. Buchwald, J. Am. Chem. Soc. 2000, 122, 12051.
- 7T. Saget, S. J. Lemouzy, N. Cramer, Angew. Chem. Int. Ed. 2012, 51, 2238; Angew. Chem. 2012, 124, 2281.
- 8W. Tang, X. Wei, W. Li, A. White, N. D. Patel, J. Savoie, J. J. Gao, S. Rodriguez, B. Qu, N. Haddad, B. Z. Lu, D. Krishnamurthy, N. K. Yee, C. H. Senanayake, Angew. Chem. Int. Ed. 2010, 49, 5879; Angew. Chem. 2010, 122, 6015.
- 9
- 9aT. Hamada, S. L. Buchwald, Org. Lett. 2002, 4, 999;
- 9bB. Saha, T. V. RajanBabu, J. Org. Chem. 2007, 72, 2357;
- 9cA. M. Taylor, R. A. Altman, S. L. Buchwald, J. Am. Chem. Soc. 2009, 131, 9900;
- 9dS. Rousseaux, J. García-Fortanet, M. A. Del Aguila Sanchez, S. L. Buchwald, J. Am. Chem. Soc. 2011, 133, 9282;
- 9eS. Lühr, J. Holz, A. Börner, ChemCatChem 2011, 3, 1708;
- 9fM. M. Pereira, M. J. F. Calvete, R. M. B. Carrilhoa, A. R. Abreu, Chem. Soc. Rev. 2013, 42, 6990;
- 9gD. Grosheva, N. Cramer, ACS Catal. 2017, 7, 7417.
- 10
- 10aB. L. Feringa, Acc. Chem. Res. 2000, 33, 346;
- 10bI. A. Shuklov, N. V. Dubrovina, H. Jiao, A. Spannenberg, A. Börner, Eur. J. Org. Chem. 2010, 1669;
- 10cE. Raluy, O. Pamies, M. Dieguez, Adv. Synth. Catal. 2009, 351, 1648;
- 10dK. N. Gavrilov, S. V. Zheglov, E. A. Rastorguev, N. N. Groshkin, M. G. Maksimova, E. B. Benetsky, V. A. Davankov, M. T. Reetz, Adv. Synth. Catal. 2010, 352, 2599.
- 11
- 11aY.-N. Ma, S.-X. Li, S.-D. Yang, Acc. Chem. Res. 2017, 50, 1480;
- 11bZ. Zhang, P. H. Dixneuf, J.-F. Soule, Chem. Commun. 2018, 54, 7265.
- 12
- 12aD. Gwon, S. Park, S. Chang, Tetrahedron 2015, 71, 4504;
- 12bZ.-Q. Lin, W.-Z. Wang, S.-B. Yan, W.-L. Duan, Angew. Chem. Int. Ed. 2015, 54, 6265; Angew. Chem. 2015, 127, 6363;
- 12cL. Liu, A.-A. Zhang, Y. Wang, F. Zhang, Z. Zuo, W.-X. Zhao, C.-L. Feng, W. Ma, Org. Lett. 2015, 17, 2046;
- 12dG. Xu, M. Li, S. Wang, W. Tang, Org. Chem. Front. 2015, 2, 1342;
- 12eZ.-J. Du, J. Guan, G.-J. Wu, P. Xu, L.-X. Gao, F.-S. Han, J. Am. Chem. Soc. 2015, 137, 632;
- 12fY. Sun, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 364; Angew. Chem. 2017, 129, 370;
- 12gS.-X. Li, Y.-N. Ma, S.-D. Yang, Org. Lett. 2017, 19, 1842;
- 12hY.-S. Jang, M. Dieckmann, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 15088; Angew. Chem. 2017, 129, 15284;
- 12iY.-M. Cui, Y. Lin, L.-W. Xu, Coord. Chem. Rev. 2017, 330, 37;
- 12jZ. Wang, T. Hayashi, Angew. Chem. Int. Ed. 2018, 57, 1702; Angew. Chem. 2018, 130, 1718;
- 12kY. Sun, N. Cramer, Chem. Sci. 2018, 9, 2981.
- 13For recent reviews on enantioselective C−H functionalizations, see:
- 13aR. Giri, B.-F. Shi, K. M. Engle, N. Maugel, J.-Q. Yu, Chem. Soc. Rev. 2009, 38, 3242;
- 13bJ. Wencel-Delord, F. Colobert, Chem. Eur. J. 2013, 19, 14010;
- 13cC. Zheng, S.-L. You, RSC Adv. 2014, 4, 6173;
- 13dD.-W. Gao, J. Zheng, K.-Y. Ye, C. Zheng, S.-L. You in Asymmetric Functionalization of C−H Bonds (Ed.: ), Royal Society of Chemistry, Cambridge, UK, 2015, p. 141;
10.1039/9781782621966-00141 Google Scholar
- 13eC. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908;
- 13fT. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu, J.-Q. Yu, Science 2018, 359, 759.
- 14For reviews, see:
- 14aB. Ye, N. Cramer, Acc. Chem. Res. 2015, 48, 1308;
- 14bC. G. Newton, D. Kossler, N. Cramer, J. Am. Chem. Soc. 2016, 138, 3935; seminal reports detailing the ligand synthesis:
- 14cB. Ye, N. Cramer, Science 2012, 338, 504;
- 14dB. Ye, N. Cramer, J. Am. Chem. Soc. 2013, 135, 636;
- 14eJ. Zheng, W.-J. Cui, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5242;
- 14fZ.-J. Jia, C. Merten, R. Gontla, C. G. Daniliuc, A. P. Antonchick, H. Waldmann, Angew. Chem. Int. Ed. 2017, 56, 2429; Angew. Chem. 2017, 129, 2469;
- 14gS. Wang, S. Hwan Park, N. Cramer, Angew. Chem. Int. Ed. 2018, 57, 5459; Angew. Chem. 2018, 130, 5557;
- 14hE. A. Trifonova, N. M. Ankudinov, A. A. Mikhaylov, D. A. Chusov, Y. V. Nelyubina, D. S. Perekalin, Angew. Chem. Int. Ed. 2018, 57, 7714; Angew. Chem. 2018, 130, 7840.
- 15Z. Liu, J.-Q. Wu, S.-D. Yang, Org. Lett. 2017, 19, 5434.
- 16S.-S. Zhang, C.-Y. Jiang, J.-Q. Wu, X.-G. Liu, Q. Li, Z.-S. Huang, D. Li, H. Wang, Chem. Commun. 2015, 51, 10240.
- 17C−H functionalization forges the chiral axis:
- 17aK. Yamaguchi, J. Yamaguchi, A. Studer, K. Itami, Chem. Sci. 2012, 3, 2165;
- 17bK. Yamaguchi, H. Kondo, J. Yamaguchi, K. Itami, Chem. Sci. 2013, 4, 3753;
- 17cY. Nishimoto, H. Kondo, K. Yamaguchi, D. Yokogawa, J. Yamaguchi, K. Itami, S. Irle, J. Org. Chem. 2017, 82, 4900; C. G. Newton, E. Braconi, J. Kuziola, M. D. Wodrich, N. Cramer, Angew. Chem. Int. Ed. 2018, Angew. Chem. Int. Ed. 2018, 57, 11040; Angew. Chem. 2018, 130, 11206.
- 18C−H functionalization locks an existing axis:
- 18aC. He, M. Hou, Z. Zhu, Z. Gu, ACS Catal. 2017, 7, 5316;
- 18bQ.-J. Yao, S. Zhang, B.-B. Zhan, B.-F. Shi, Angew. Chem. Int. Ed. 2017, 56, 6617; Angew. Chem. 2017, 129, 6717;
- 18cG. Liao, Q.-J. Yao, Z.-Z. Zhang, Y.-J. Wu, D.-Y. Huang, B.-F. Shi, Angew. Chem. Int. Ed. 2018, 57, 3665; Angew. Chem. 2018, 130, 3723.;
- 18dF. Kakiuchi, P. Le Gendre, A. Yamada, H. Ohtaki, S. Murai, Tetrahedron: Asymmetry 2000, 11, 2647;
- 18eJ. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2014, 53, 13244; Angew. Chem. 2014, 126, 13460.
- 19
- 19aG. Bringmann, A. J. P. Mortimer, P. A. Keller, M. J. Gresser, J. Garner, M. Breuning, Angew. Chem. Int. Ed. 2005, 44, 5384; Angew. Chem. 2005, 117, 5518;
- 19bP. Loxq, E. Manoury, R. Poli, E. Deydier, A. Labande, Coord. Chem. Rev. 2016, 308, 131;
- 19cB. Zilate, A. Castrogiovanni, C. Sparr, ACS Catal. 2018, 8, 2981;
- 19dA. Link, C. Sparr, Chem. Soc. Rev. 2018, 47, 3804.
- 20Compounds 3 aa–3 la and 3 ba–3 bg appear as a set of rotamers by NMR spectroscopy.
- 21CCDC 1854285, 1854287, and 1854288 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 22T. Imamoto, S.-I. Kikuchi, T. Miura, Y. Wada, Org. Lett. 2001, 3, 87.