Synthesis of Fucosylated Chondroitin Sulfate Nonasaccharide as a Novel Anticoagulant Targeting Intrinsic Factor Xase Complex
Xiao Zhang
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorHuiying Liu
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorLisha Lin
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201 China
Search for more papers by this authorWang Yao
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorProf. Jinhua Zhao
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201 China
Search for more papers by this authorCorresponding Author
Dr. Mingyi Wu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhongjun Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorXiao Zhang
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorHuiying Liu
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorLisha Lin
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201 China
Search for more papers by this authorWang Yao
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorProf. Jinhua Zhao
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201 China
Search for more papers by this authorCorresponding Author
Dr. Mingyi Wu
State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming, 650201 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhongjun Li
State Key Laboratory of Natural and Biomimetic Drugs, Department of Chemical Biology, School of Pharmaceutical Sciences, Peking University, Beijing, 100191 China
Search for more papers by this authorGraphical Abstract
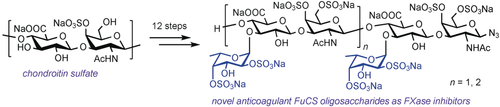
To the nines: Structurally defined fucosylated chondroitin sulfate hexa- and nonasaccharides were synthesized in 12 linear steps from chondroitin sulfate through the enzymatic degradation of chondroitin (see scheme). The nonasaccharide displayed selective intrinsic factor Xase complex inhibitory activity by binding to factor IXa with high affinity, thus showing promise for the development of anticoagulant agents targeting the intrinsic coagulation pathway.
Abstract
Fucosylated chondroitin sulfate (FuCS) is a structurally distinct glycosaminoglycan, and its oligosaccharides exhibit excellent anticoagulant activity with lower risks of adverse effects and bleeding. Herein we report a facile approach to the synthesis of FuCS hexa- and nonasaccharides on the basis of the enzymatic degradation of chondroitin over 12 linear steps. As compared with a clinical low-molecular-weight heparin drug (enoxaparin), the nonasaccharide synthesized in this study displayed similar APTT activity and selective intrinsic factor Xase complex inhibitory activity ((12.9±0.83) nm) by binding to factor IXa with high affinity, thus offering promise for the development of new anticoagulant agents targeting the intrinsic coagulation pathway.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201807546-sup-0001-misc_information.pdf6.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aN. Mackman, Nature 2008, 451, 914–918;
- 1bE. F. Plow, Y. Wang, D. I. Simon, Blood 2018, 131, 1899–1902.
- 2H. Brandstetter, M. Bauer, R. Huber, P. Lollar, W. Bode, Proc. Natl. Acad. Sci. USA 1995, 92, 9796–9800.
- 3J. P. Sheehan, E. N. Walke, Blood 2006, 107, 3876–3882.
- 4V. H. Pomin, Mar. Drugs 2014, 12, 232–254.
- 5L. Zhao, M. Wu, C. Xiao, L. Yang, L. Zhou, N. Gao, Z. Li, J. Chen, J. Chen, J. Liu, H. Qin, J. Zhao, Proc. Natl. Acad. Sci. USA 2015, 112, 8284–8289.
- 6
- 6aF. Shang, N. Gao, R, Yin, L. Lin, C. Xiao, L. Zhou, Z. Li, S. W. Purcell, M. Wu, J. Zhao, Eur. J. Med. Chem. 2018, 148, 423–435;
- 6bM. Wu, D. Wen, N. Gao, C. Xiao, L. Yang, L. Xu, W. Lian, W. Peng, J. Jiang, J. Zhao, Eur. J. Med. Chem. 2015, 92, 257–269;
- 6cL. Yan, J. Li, D. Wang, T. Ding, Y. Hu, X. Ye, R. J. Linhardt, S. Chen, Carbohydr. Polym. 2017, 178, 180–189.
- 7J.-I. Tamura, H. Tanaka, A. Nakamura, N. Takeda, Tetrahedron Lett. 2013, 54, 3940–3943.
- 8
- 8aA. Laezza, A. Iadonisi, C. D. Castro, M. D. Rosa, C. Schiraldi, M. Parrilli, E. Bedini, Biomacromolecules 2015, 16, 2237–2245;
- 8bA. Laezza, A. Iadonisi, A. V. A. Pirozzi, P. Diana, M. D. Rosa, C. Schiraldi, M. Parrilli, E. Bedini, Chem. Eur. J. 2016, 22, 18215–18226.
- 9X. Zhang, W. Yao, X. Xu, H. Sun, J. Zhao, X. Meng, M. Wu, Z. Li, Chem. Eur. J. 2018, 24, 1694–1700.
- 10
- 10aM. Mende, C. Bednarek, M. Wawryszyn, P. Sauter, M. B. Biskup, U. Schepers, S. Brase, Chem. Rev. 2016, 116, 8193–8255;
- 10bW. Lu, C. Zong, P. Chopra, L. E. Pepi, Y. Xu, I. J. Amster, J. Liu, G.-J. Boons, Angew. Chem. Int. Ed. 2018, 57, 5340–5344; Angew. Chem. 2018, 130, 5438–5442;
- 10cJ. Li, G. Su, J. Liu, Angew. Chem. Int. Ed. 2017, 56, 11784–11787; Angew. Chem. 2017, 129, 11946–11949;
- 10dY. Maki, R. Okamoto, M. Izumi, T. Murase, Y. Kajihara, J. Am. Chem. Soc. 2016, 138, 3461–3468;
- 10eC. Solera, G. Macchione, S. Maza, M. M. Kayser, F. Corzana, J. L. de Paz, P. M. Nieto, Chem. Eur. J. 2016, 22, 2356–2369;
- 10fC.-H. Chang, L. S. Lico, T.-Y. Huang, S.-Y. Lin, C.-L. Chang, S. D. Arco, S.-C. Hung, Angew. Chem. Int. Ed. 2014, 53, 9876–9879; Angew. Chem. 2014, 126, 10034–10037;
- 10gK. Yoshida, B. Yang, W. Yang, Z. Zhang, J. Zhang, X. Huang, Angew. Chem. Int. Ed. 2014, 53, 9051–9058; Angew. Chem. 2014, 126, 9197–9204;
- 10hS. Eller, M. Collot, J. Yin, H. S. Hahm, P. H. Seeberger, Angew. Chem. Int. Ed. 2013, 52, 5858–5861; Angew. Chem. 2013, 125, 5970–5973;
- 10iY. I. Oh, G. J. Sheng, S.-K. Chang, L. C. Hsieh-Wilson, Angew. Chem. Int. Ed. 2013, 52, 11796–11799; Angew. Chem. 2013, 125, 12012–12015.
- 11
- 11aP. P. Deshpande, S. J. Danishefsky, Nature 1997, 387, 164–166;
- 11bP. P. Deshpande, H. M. Kim, A. Zatorski, T.-K. Park, G. Ragupathi, P. O. Livingston, D. Live, S. J. Danishefsky, J. Am. Chem. Soc. 1998, 120, 1600–1614;
- 11cQ. Li, Z. Guo, Org. Lett. 2017, 19, 6558–6561.
- 12
- 12aS. Kobayashi, H. Morii, R. Itoh, S. Kimura, M. Ohmae, J. Am. Chem. Soc. 2001, 123, 11825–11826;
- 12bS. Kobayashi, S.-I. Fujikawa, M. Ohmae, J. Am. Chem. Soc. 2003, 125, 14357–14369.
- 13I. Kakizaki, H. Koizumi, F. Chen, M. Endo, Carbohydr. Polym. 2015, 121, 362–371.
- 14
- 14aX. Lu, X. Huang, Glycoconjugate J. 2015, 32, 549–556;
- 14bS. Köhling, G. Künze, K. Lemmnitzer, M. Bermudez, G. Wolber, J. Schiller, D. Huster, J. Rademann, Chem. Eur. J. 2016, 22, 5563–5574;
- 14cS. Köhling, M. P. Exner, S. Nojoumi, J. Schiller, N. Budisa, J. Rademann, Angew. Chem. Int. Ed. 2016, 55, 15510–15514; Angew. Chem. 2016, 128, 15736–15740;
- 14dM. Sha, W. Yao, X. Zhang, Z. Li, Tetrahedron Lett. 2017, 58, 2910–2914.
- 15J. J. Lim, J. S. Temenoff, Biomaterials 2013, 34, 5007–5018.
- 16T. Tanaka, H. Nagai, M. Noguchi, A. Kobayashi, S.-I. Shoda, Chem. Commun. 2009, 23, 3378–3379.
- 17C. Lopin-Bon, J.-C. Jacquinet, Angew. Chem. Int. Ed. 2006, 45, 2574–2578; Angew. Chem. 2006, 118, 2636–2640.
- 18Y. Zhou, M. Rahm, B. Wu, X. Zhang, B. Ren, H. Dong, J. Org. Chem. 2013, 78, 11618–11622.
- 19J. Zhou, B. Gao, Zh. Xu, T. Ye, J. Am. Chem. Soc. 2016, 138, 6948–6951.
- 20A. V. Kornilov, E. V. Sukhova, N. E. Nifantiev, Carbohydr. Res. 2001, 336, 309–313.
- 21
- 21aJ. Zhou, L. Yang, W. Hu, J. Org. Chem. 2014, 79, 4718–4726;
- 21bH. Tanaka, T. Ishida, N. Matoba, H. Tsukamoto, H. Yamada, T. Takahashi, Angew. Chem. Int. Ed. 2006, 45, 6349–6352; Angew. Chem. 2006, 118, 6497–6500.
- 22A. Cheng, J. L. Hendel, K. Colangelo, M. Bonin, F. I. Auzanneau, J. Org. Chem. 2008, 73, 7574–7579.
- 23
- 23aM. Guillemineau, F.-I. Auzanneau, Carbohydr. Res. 2012, 357, 132–138;
- 23bC. Filser, D. Kowalczyk, C. Jones, M. K. Wild, U. Ipe, D. Vestweber, H. Kunz, Angew. Chem. Int. Ed. 2007, 46, 2108–2111; Angew. Chem. 2007, 119, 2155–2159.
- 24F. P. Boulineau, A. Wei, J. Org. Chem. 2004, 69, 3391–3399.
- 25A. Koizumi, I. Matsuo, M. Takatani, A. Seko, M. Hachisu, Y. Takeda, Y. Ito, Angew. Chem. Int. Ed. 2013, 52, 7426–7431; Angew. Chem. 2013, 125, 7574–7579.
- 26J.-C. Jacquinet, C. Lopin-Bon, A. Vibert, Chem. Eur. J. 2009, 15, 9579–9595.