Modular Redesign of a Cationic Lytic Peptide To Promote the Endosomal Escape of Biomacromolecules
Dr. Yusuke Azuma
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorHaruka Imai
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorDr. Yoshimasa Kawaguchi
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorProf. Dr. Ikuhiko Nakase
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorProf. Dr. Hiroshi Kimura
Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Shiroh Futaki
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorDr. Yusuke Azuma
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorHaruka Imai
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorDr. Yoshimasa Kawaguchi
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorProf. Dr. Ikuhiko Nakase
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorProf. Dr. Hiroshi Kimura
Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama, 226-8503 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Shiroh Futaki
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611-0011 Japan
Search for more papers by this authorGraphical Abstract
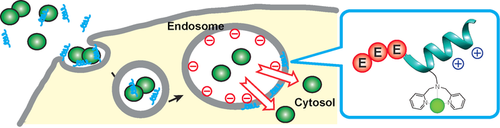
Break the trap: Inefficient endosomal escape into the cytosol has been a bottleneck in intracellular delivery of biomacromolecules. With appropriate chemical modifications, a cationic amphiphilic peptide, Mastoparan X, became a useful delivery tool that selectively disrupts the endosomal membranes and releases entrapped materials.
Abstract
Endocytosis is an important route for the intracellular delivery of biomacromolecules, wherein their inefficient endosomal escape into the cytosol remains a major barrier. Based on the understanding that endosomal membranes are negatively charged, we focused on the potential of cationic lytic peptides for developing endosomolysis agents to release such entrapped molecules. As such, a venom peptide, Mastoparan X, was employed and redesigned to serve as a delivery tool. Appending a tri-glutamate unit to the N-terminus attenuates the cytotoxicity of Mastoparan X by about 40 fold, while introduction of a NiII-dipicolylamine complex enhances cellular uptake of the peptide by about 17 fold. Using the optimized peptide, various fluorescently labeled macromolecules were successfully delivered to the cytosol, enabling live-cell imaging of acetylated histones.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201807534-sup-0001-misc_information.pdf4.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. Guillard, R. R. Minter, R. H. Jackson, Trends Biotechnol. 2015, 33, 163–171;
- 1bD. Schumacher, J. Helma, A. F. L. Schneider, H. Leonhardt, C. P. R. Hackenberger, Angew. Chem. Int. Ed. 2018, 57, 2314–2333; Angew. Chem. 2018, 130, 2336–2357;
- 1cY. Zhang, J. J. Røise, K. Lee, J. Li, N. Murthy, Curr. Opin. Biotechnol. 2018, 52, 25–31.
- 2G. J. Doherty, H. T. McMahon, Annu. Rev. Biochem. 2009, 78, 857–902.
- 3J. Huotari, A. Helenius, EMBO J. 2011, 30, 3481–3500.
- 4
- 4aN. K. Subbarao, R. A. Parente, F. C. Szoka, L. Nadasdi, K. Pongracz, Biochemistry 1987, 26, 2964–2972;
- 4bS. A. Wharton, S. R. Martin, R. W. H. Ruigrok, J. J. Skehel, D. C. Wiley, J. Gen. Virol. 1988, 69, 1847–1857;
- 4cW. Li, F. Nicol, F. C. Szoka, Adv. Drug Delivery Rev. 2004, 56, 967–985;
- 4dJ. S. Wadia, R. V. Stan, S. F. Dowdy, Nat. Med. 2004, 10, 310;
- 4eY. Yamada, Y. Shinohara, T. Kakudo, S. Chaki, S. Futaki, H. Kamiya, H. Harashima, Int. J. Pharm. 2005, 303, 1–7;
- 4fS. Kobayashi, I. Nakase, N. Kawabata, H.-H. Yu, S. Pujals, M. Imanishi, E. Giralt, S. Futaki, Bioconjugate Chem. 2009, 20, 953–959;
- 4gT. Yoshida, T. C. Lai, G. S. Kwon, K. Sako, Expert Opin. Drug Delivery 2013, 10, 1497–1513;
- 4hS. Bazban-Shotorbani, M. M. Hasani-Sadrabadi, A. Karkhaneh, V. Serpooshan, K. I. Jacob, A. Moshaverinia, M. Mahmoudi, J. Controlled Release 2017, 253, 46–63;
- 4iG. Kocak, C. Tuncer, V. Butun, Polym. Chem. 2017, 8, 144–176;
- 4jG. Wiedman, S. Y. Kim, E. Zapata-Mercado, W. C. Wimley, K. Hristova, J. Am. Chem. Soc. 2017, 139, 937–945.
- 5
- 5aT. Kobayashi, E. Stang, K. S. Fang, P. de Moerloose, R. G. Parton, J. Gruenberg, Nature 1998, 392, 193;
- 5bW. Möbius, E. Van Donselaar, Y. Ohno-Iwashita, Y. Shimada, H. F. G. Heijnen, J. W. Slot, H. J. Geuze, Traffic 2003, 4, 222–231.
- 6G. van Meer, D. R. Voelker, G. W. Feigenson, Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124.
- 7
- 7aS.-T. Yang, E. Zaitseva, L. V. Chernomordik, K. Melikov, Biophys. J. 2010, 99, 2525–2533;
- 7bA. Erazo-Oliveras, K. Najjar, D. Truong, T.-Y. Wang, D. J. Brock, A. R. Prater, J.-P. Pellois, Cell Chem. Biol. 2016, 23, 598–607;
- 7cZ. Qian, A. Martyna, R. L. Hard, J. Wang, G. Appiah-Kubi, C. Coss, M. A. Phelps, J. S. Rossman, D. Pei, Biochemistry 2016, 55, 2601–2612.
- 8K. Matsuzaki, Biochim. Biophys. Acta Biomembr. 1999, 1462, 1–10.
- 9
- 9aY. Todokoro, I. Yumen, K. Fukushima, S.-W. Kang, J.-S. Park, T. Kohno, K. Wakamatsu, H. Akutsu, T. Fujiwara, Biophys. J. 2006, 91, 1368–1379;
- 9bY. Hirai, T. Yasuhara, H. Yoshida, T. Nakajima, M. Fujino, C. Kitada, Chem. Pharm. Bull. (Tokyo) 1979, 27, 1942–1944;
- 9cK. Matsuzaki, S. Yoneyama, O. Murase, K. Miyajima, Biochemistry 1996, 35, 8450–8456.
- 10R. S. Signarvic, W. F. DeGrado, J. Am. Chem. Soc. 2009, 131, 3377–3384.
- 11
- 11aS. Frey, L. K. Tamm, Biophys. J. 1991, 60, 922–930;
- 11bB. Bechinger, J. Membr. Biol. 1997, 156, 197–211;
- 11cD. Sengupta, L. Meinhold, D. Langosch, G. M. Ullmann, C. Smith Jeremy, Proteins Struct. Funct. Bioinf. 2005, 58, 913–922.
- 12H. Lecoeur, Exp. Cell Res. 2002, 277, 1–14.
- 13
- 13aD. R. Rice, K. J. Clear, B. D. Smith, Chem. Commun. 2016, 52, 8787–8801;
- 13bT. Sakamoto, A. Ojida, I. Hamachi, Chem. Commun. 2009, 141–152;
- 13cJ. R. Johnson, H. Jiang, B. D. Smith, Bioconjugate Chem. 2008, 19, 1033–1039;
- 13dV. Yarlagadda, P. Sarkar, S. Samaddar, J. Haldar, Angew. Chem. Int. Ed. 2016, 55, 7836–7840; Angew. Chem. 2016, 128, 7967–7971.
- 14A. Ojida, Y. Mito-oka, K. Sada, I. Hamachi, J. Am. Chem. Soc. 2004, 126, 2454–2463.
- 15Y. Azuma, H. Imai, T. Yoshimura, T. Kawabata, M. Imanishi, S. Futaki, Org. Biomol. Chem. 2012, 10, 6062–6068.
- 16Metal-binding to the Dpa moiety of the peptide resulted in significant fluorescence quenching of Alexa488 (Supporting Information, Figure S4A). The relative cellular uptakes shown in Figure 2 C were obtained by multiplying the observed fluorescent values by the quenching factors, while the data without these processes are also provided in the Supporting Information, Figure S4B.
- 17Endocytosis was confirmed to be a major route for the cytosolic appearance of FITC-dextran by microscopy time-course and the experiment at 4 °C (Supporting Information, Figure S6,S7).
- 18M. V. Berridge, P. M. Herst, A. S. Tan, Biotechnol. Annu. Rev. 2005, 11, 127–152.
- 19M. Smolarsky, D. Teitelbaum, M. Sela, C. Gitler, J. Immunol. Methods 1977, 15, 255–265.
- 20
- 20aR. E. Chapman, S. Munro, EMBO J. 1994, 13, 2305–2312;
- 20bR. Puertollano, M. A. Alonso, Mol. Biol. Cell 1999, 10, 3435–3447.
- 21Y. Hayashi-Takanaka, K. Yamagata, T. Wakayama, T. J. Stasevich, T. Kainuma, T. Tsurimoto, M. Tachibana, Y. Shinkai, H. Kurumizaka, N. Nozaki, H. Kimura, Nucleic Acids Res. 2011, 39, 6475–6488.
- 22
- 22aY. Hayashi-Takanaka, K. Yamagata, N. Nozaki, H. Kimura, J. Cell Biol. 2009, 187, 781–790;
- 22bH. Kimura, J. Hum. Genet. 2013, 58, 439;
- 22cY. Hayashi-Takanaka, T. J. Stasevich, H. Kurumizaka, N. Nozaki, H. Kimura, PLoS One 2014, 9, e 106271.
- 23M. Akishiba, T. Takeuchi, Y. Kawaguchi, K. Sakamoto, H.-H. Yu, I. Nakase, T. Takatani-Nakase, F. Madani, A. Gräslund, S. Futaki, Nat. Chem. 2017, 9, 751.