Site-Selective Remote Radical C−H Functionalization of Unactivated C−H Bonds in Amides Using Sulfone Reagents
Dr. Yong Xia
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
These authors contributed equally to this work.
Search for more papers by this authorLin Wang
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Armido Studer
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorDr. Yong Xia
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
These authors contributed equally to this work.
Search for more papers by this authorLin Wang
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
These authors contributed equally to this work.
Search for more papers by this authorCorresponding Author
Prof. Dr. Armido Studer
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität, Corrensstrasse 40, 48149 Münster, Germany
Search for more papers by this authorGraphical Abstract
Abstract
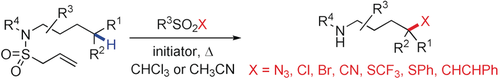
A general and practical strategy for remote site-selective functionalization of unactivated aliphatic C−H bonds in various amides by radical chemistry is introduced. C−H bond functionalization is achieved by using the readily installed N-allylsulfonyl moiety as an N-radical precursor. The in situ generated N-radical engages in intramolecular 1,5-hydrogen atom transfer to generate a translocated C radical which is subsequently trapped with various sulfone reagents to afford the corresponding C−H functionalized amides. The generality of the approach is documented by the successful remote C−N3, C−Cl, C−Br, C−SCF3, C−SPh, and C−C bond formation. Unactivated tertiary and secondary C−H bonds, as well as activated primary C−H bonds, can be readily functionalized by this method.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201807455-sup-0001-misc_information.pdf19 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. Yuan, C. Liu, A. Lei, Chem. Commun. 2015, 51, 1394;
- 1bJ. F. Hartwig, M. A. Larsen, ACS Cent. Sci. 2016, 2, 281;
- 1cJ. C. K. Chu, T. Rovis, Angew. Chem. Int. Ed. 2018, 57, 62; Angew. Chem. 2018, 130, 64.
- 2
- 2aH. Yi, G. Zhang, H. Wang, Z. Huang, J. Wang, A. K. Singh, A. Lei, Chem. Rev. 2017, 117, 9016;
- 2bD. Ravelli, M. Fagnoni, T. Fukuyama, T. Nishikawa, I. Ryu, ACS Catal. 2018, 8, 701.
- 3
- 3aL. M. Stateman, K. M. Nakafuku, D. A. Nagib, Synthesis 2018, 50, 1569;
- 3bJ. Robertson, J. Pillai, R. K. Lush, Chem. Soc. Rev. 2001, 30, 94;
- 3cS. Chiba, H. Chen, Org. Biomol. Chem. 2014, 12, 4051;
- 3dS. Z. Zard, Chem. Soc. Rev. 2008, 37, 1603.
- 4
- 4aK. Löffler, C. Freytag, Ber. Dtsch. Chem. Ges. 1909, 42, 3427;
10.1002/cber.19090420377 Google Scholar
- 4bA. W. Hofmann, Ber. Dtsch. Chem. Ges. 1883, 16, 558;
10.1002/cber.188301601120 Google Scholar
- 4cP. Mackiewicz, R. Furstoss, Tetrahedron 1978, 34, 3241;
- 4dR. S. Neale, Synthesis 1971, 1;
- 4eM. E. Wolff, Chem. Rev. 1963, 63, 55.
- 5
- 5aQ. Qin, S. Yu, Org. Lett. 2015, 17, 1894;
- 5bT. Liu, T.-S. Mei, J.-Q. Yu, J. Am. Chem. Soc. 2015, 137, 5871;
- 5cJ. Richers, M. Heilmann, M. Drees, K. Tiefenbacher, Org. Lett. 2016, 18, 6472;
- 5dE. A. Wappes, S. C. Fosu, T. C. Chopko, D. A. Nagib, Angew. Chem. Int. Ed. 2016, 55, 9974; Angew. Chem. 2016, 128, 10128.
- 6For elegant site-selective, remote C−C bond formation, see:
- 6aG. J. Choi, Q. Zhu, D. C. Miller, C. J. Gu, R. R. Knowles, Nature 2016, 539, 268;
- 6bJ. C. K. Chu, T. Rovis, Nature 2016, 539, 272;
- 6cW. Yuan, Z. Zhou, L. Gong, E. Meggers, Chem. Commun. 2017, 53, 8964;
- 6dD.-F. Chen, J. C. K. Chu, T. Rovis, J. Am. Chem. Soc. 2017, 139, 14897;
- 6eH. Jiang, A. Studer, Angew. Chem. Int. Ed. 2017, 56, 12273; Angew. Chem. 2017, 129, 12441.
- 7C. Moutrille, S. Z. Zard, Chem. Commun. 2004, 1848.
- 8
- 8aX. Huang, J. T. Groves, ACS Catal. 2016, 6, 751;
- 8bS. Bräse, C. Gil, K. Knepper, V. Zimmermann, Angew. Chem. Int. Ed. 2005, 44, 5188; Angew. Chem. 2005, 117, 5320;
- 8cE. Lallana, R. Riguera, E. Fernandez-Megia, Angew. Chem. Int. Ed. 2011, 50, 8794; Angew. Chem. 2011, 123, 8956;
- 8dC. I. Schilling, N. Jung, M. Biskup, U. Schepers, S. Brase, Chem. Soc. Rev. 2011, 40, 4840;
- 8eE. M. Sletten, C. R. Bertozzi, Acc. Chem. Res. 2011, 44, 666.
- 9
- 9aP. Panchaud, L. Chabaud, Y. Landais, C. Ollivier, P. Renaud, S. Zigmantas, Chem. Eur. J. 2004, 10, 3606;
- 9bE. Nyfeler, P. Renaud, Org. Lett. 2008, 10, 985;
- 9cA. Kapat, A. König, F. Montermini, P. Renaud, J. Am. Chem. Soc. 2011, 133, 13890;
- 9dY. Zhu, X. Li, X. Wang, X. Huang, T. Shen, Y. Zhang, X. Sun, M. Zou, S. Song, N. Jiao, Org. Lett. 2015, 17, 4702;
- 9eC. Liu, X. Wang, Z. Li, L. Cui, C. Li, J. Am. Chem. Soc. 2015, 137, 9280.
- 10
- 10aV. V. Zhdankin, C. J. Kuehl, A. P. Krasutsky, M. S. Formaneck, J. T. Bolz, Tetrahedron Lett. 1994, 35, 9677;
- 10bV. V. Zhdankin, A. P. Krasutsky, C. J. Kuehl, A. J. Simonsen, J. K. Woodward, B. Mismash, J. T. Bolz, J. Am. Chem. Soc. 1996, 118, 5192;
- 10cR. R. Karimov, A. Sharma, J. F. Hartwig, ACS Cent. Sci. 2016, 2, 715;
- 10dP. T. G. Rabet, G. Fumagalli, S. Boyd, M. F. Greaney, Org. Lett. 2016, 18, 1646.
- 11T. R. Belliotti, T. Capiris, I. V. Ekhato, J. J. Kinsora, M. J. Field, T. G. Heffner, L. T. Meltzer, et al., J. Med. Chem. 2005, 48, 2294.
- 12
- 12aT. D. Aicher, R. E. Damon, J. Koletar, C. C. Vinluan, L. J. Brand, J. P. Gao, S. S. Shetty, et al., Bioorg. Med. Chem. Lett. 1999, 9, 2223;
- 12bM. Pirttimaa, A. Nasereddin, D. Kopelyanskiy, M. Kaiser, J. Yli-Kauhaluoma, K.-M. Oksman-Caldentey, R. Brun, et al., J. Nat. Prod. 2016, 79, 362.
- 13
- 13aV. Agarwal, Z. D. Miles, J. M. Winter, A. S. Eustaquio, A. A. El Gamal, B. S. Moore, Chem. Rev. 2017, 117, 5619;
- 13bD. A. Petrone, J. Ye, M. Lautens, Chem. Rev. 2016, 116, 8003.
- 14
- 14aX. Xu, K. Matsuzaki, N. Shibata, Chem. Rev. 2015, 115, 731;
- 14bX. Yang, T. Wu, R. J. Phipps, F. D. Toste, Chem. Rev. 2015, 115, 826;
- 14cC. Ni, M. Hu, J. Hu, Chem. Rev. 2015, 115, 765;
- 14dP. Chauhan, S. Mahajan, D. Enders, Chem. Rev. 2014, 114, 8807.
- 15For using PhSO2SCF3 as the SCF3 source, see:
- 15aH. Li, C. Shan, C.-H. Tung, Z. Xu, Chem. Sci. 2017, 8, 2610;
- 15bX. Zhao, B. Yang, A. Wei, J. Sheng, M. Tian, Q. Li, K. Lu, Tetrahedron Lett. 2018, 59, 1719.
- 16For selected examples using PhSO2SPh as the SPh source, see:
- 16aM. Kobayashi, M. Kobayashi, M. Yoshida, Bull. Chem. Soc. Jpn. 1985, 58, 473;
- 16bS. Kim, S. Kim, N. Otsuka, I. Ryu, Angew. Chem. Int. Ed. 2005, 44, 6183; Angew. Chem. 2005, 117, 6339;
- 16cW. Kong, H. An, Q. Song, Chem. Commun. 2017, 53, 8968;
- 16dA.-P. Schaffner, F. Montermini, D. Pozzi, V. Darmency, E. M. Scanlan, P. Renaud, Adv. Synth. Catal. 2008, 350, 1163.
- 17
- 17aJ.-M. Brunel, I. P. Holmes, Angew. Chem. Int. Ed. 2004, 43, 2752; Angew. Chem. 2004, 116, 2810;
- 17bY. Ping, Q. Ding, Y. Peng, ACS Catal. 2016, 6, 5989.
- 18For selected examples using TsCN as the CN source, see:
- 18aJ.-M. Fang, M.-Y. Chen, Tetrahedron Lett. 1987, 28, 2853;
- 18bS. Kamijo, T. Hoshikawa, M. Inoue, Org. Lett. 2011, 13, 5928;
- 18cJ. Sun, P. Li, L. Guo, F. Yu, Y.-P. He, L. Chu, Chem. Commun. 2018, 54, 3162.
- 19Alternatively the undecyl radical derived from DLP might also directly react with substrate 1 a to initiate the chain.