Photochromic Benzo[b]phosphole Alkynylgold(I) Complexes with Mechanochromic Property to Serve as Multistimuli-Responsive Materials
Nathan Man-Wai Wu
Institute of Molecular Functional Materials, Areas of Excellence Scheme, University Grants Committee (Hong Kong) and, Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China
Search for more papers by this authorDr. Maggie Ng
Institute of Molecular Functional Materials, Areas of Excellence Scheme, University Grants Committee (Hong Kong) and, Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Vivian Wing-Wah Yam
Institute of Molecular Functional Materials, Areas of Excellence Scheme, University Grants Committee (Hong Kong) and, Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China
Search for more papers by this authorNathan Man-Wai Wu
Institute of Molecular Functional Materials, Areas of Excellence Scheme, University Grants Committee (Hong Kong) and, Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China
Search for more papers by this authorDr. Maggie Ng
Institute of Molecular Functional Materials, Areas of Excellence Scheme, University Grants Committee (Hong Kong) and, Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Vivian Wing-Wah Yam
Institute of Molecular Functional Materials, Areas of Excellence Scheme, University Grants Committee (Hong Kong) and, Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China
Search for more papers by this authorGraphical Abstract
Abstract
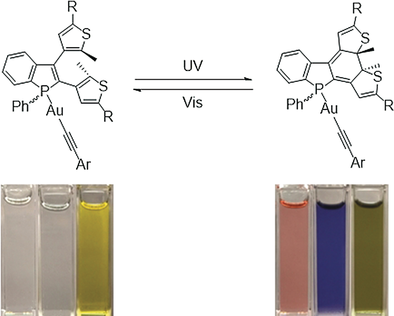
A series of novel benzo[b]phosphole alkynylgold(I) complexes has been demonstrated to display photochromic and mechanochromic properties upon applying the respective stimuli of light and mechanical force. Promising multistimuli-responsive properties of this series of gold(I) complexes have been successfully achieved through judicious molecular design, which involves incorporation of the photochromic dithienylethene-containing benzo[b]phosphole into the triphenylamine-containing arylethynyl ligand that is susceptible to mechanical force-induced color changes via gold(I) complexation. With excellent thermal irreversibility and robust fatigue resistance of this series of gold(I) complexes, multicolor states controlled by the photochromism and mechanochromism have been realized. Repeatable photochromic and mechanochromic cycles without apparent loss of reactivity have also been observed under ambient conditions. The present work provides important insight and an alternative strategy for the molecular design of multistimuli-responsive materials, paving the way for further development of the underexplored photoresponsive gold(I) complexes and the multistate photocontrolled system.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201806272-sup-0001-misc_information.pdf1.8 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. Fihey, A. Perrier, W. R. Browne, D. Jacquemin, Chem. Soc. Rev. 2015, 44, 3719.
- 2A. J. Myles, T. J. Wigglesworth, N. R. Branda, Adv. Mater. 2003, 15, 745.
- 3
- 3aE. A. Shilova, A. Heynderickx, O. Siri, J. Org. Chem. 2010, 75, 1855;
- 3bS. Chen, Y. Yang, Y. Wu, H. Tian, W. Zhu, J. Mater. Chem. 2012, 22, 5486;
- 3cS. Cui, Y. Tang, R. Lu, S. Pu, RSC Adv. 2016, 6, 107475.
- 4
- 4aA. Kishimura, T. Yamashita, K. Yamaguchi, T. Aida, Nat. Mater. 2005, 4, 546;
- 4bP. Naumov, S. Chizhik, M. K. Panda, N. K. Nath, E. Boldyreva, Chem. Rev. 2015, 115, 12440;
- 4cJ. Chen, F. K.-C. Leung, M. C. A. Stuart, T. Kajitani, T. Fukushima, E. van der Giessen, B. L. Feringa, Nat. Chem. 2018, 10, 132.
- 5
- 5aM.-M. Russew, S. Hecht, Adv. Mater. 2010, 22, 3348;
- 5bL. Zhang, X. Zhong, E. Pavlica, S. Li, A. Klekachev, G. Bratina, T. W. Ebbesen, E. Orgiu, P. Samori, Nat. Nanotechnol. 2016, 11, 900;
- 5cL. Wang, Q. Li, Chem. Soc. Rev. 2018, 47, 1044.
- 6
- 6aJ. Zhang, Q. Zou, H. Tian, Adv. Mater. 2013, 25, 378;
- 6bM. Irie, T. Fukaminato, K. Matsuda, S. Kobatake, Chem. Rev. 2014, 114, 12174;
- 6cJ. Zhang, H. Tian, Adv. Opt. Mater. 2018, 6, 1701278;
- 6dC.-C. Ko, V. W.-W. Yam, Acc. Chem. Res. 2018, 51, 149.
- 7
- 7aJ. C.-H. Chan, W. H. Lam, H.-L. Wong, W.-T. Wong, V. W.-W. Yam, Angew. Chem. Int. Ed. 2013, 52, 11504; Angew. Chem. 2013, 125, 11718;
- 7bJ. C.-H. Chan, W. H. Lam, H.-L. Wong, W.-T. Wong, V. W.-W. Yam, Chem. Eur. J. 2015, 21, 6936;
- 7cN. M.-W. Wu, H.-L. Wong, V. W.-W. Yam, Chem. Sci. 2017, 8, 1309;
- 7dN. M.-W. Wu, M. Ng, W. H. Lam, H.-L. Wong, V. W.-W. Yam, J. Am. Chem. Soc. 2017, 139, 15142.
- 8
- 8aT. Baumgartner, R. Réau, Chem. Rev. 2006, 106, 4681;
- 8bT. Baumgartner, Acc. Chem. Res. 2014, 47, 1613;
- 8cM. P. Duffy, W. Delaunay, P.-A. Bouit, M. Hissler, Chem. Soc. Rev. 2016, 45, 5296;
- 8dE. Y.-H. Hong, C.-T. Poon, V. W.-W. Yam, J. Am. Chem. Soc. 2016, 138, 6368;
- 8eS. Y.-L. Leung, S. Evariste, C. Lescop, M. Hissler, V. W.-W. Yam, Chem. Sci. 2017, 8, 4264;
- 8fA. F.-F. Cheung, E. Y.-H. Hong, V. W.-W. Yam, Chem. Eur. J. 2018, 24, 1383.
- 9
- 9aJ. C. Vickery, M. M. Olmstead, E. Y. Fung, A. L. Balch, Angew. Chem. Int. Ed. Engl. 1997, 36, 1179; Angew. Chem. 1997, 109, 1227;
- 9bH. Schmidbaur, A. Schier, Chem. Soc. Rev. 2012, 41, 370;
- 9cV. W.-W. Yam, V. K.-M. Au, S. Y.-L. Leung, Chem. Rev. 2015, 115, 7589.
- 10A. Kishimura, T. Yamashita, T. Aida, J. Am. Chem. Soc. 2005, 127, 179.
- 11
- 11aV. W.-W. Yam, C.-K. Li, C.-L. Chan, Angew. Chem. Int. Ed. Engl. 1998, 37, 2857;
10.1002/(SICI)1521-3773(19981102)37:20<2857::AID-ANIE2857>3.0.CO;2-G CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 3041–3044;10.1002/(SICI)1521-3757(19981016)110:20<3041::AID-ANGE3041>3.0.CO;2-E Web of Science® Google Scholar
- 11bX.-F. Jiang, F. K.-W. Hau, Q.-F. Sun, S.-Y. Yu, V. W.-W. Yam, J. Am. Chem. Soc. 2014, 136, 10921;
- 11cA. Chu, F. K.-W. Hau, V. W.-W. Yam, Chem. Eur. J. 2017, 23, 11076.
- 12
- 12aE. Y.-H. Hong, H.-L. Wong, V. W.-W. Yam, Chem. Commun. 2014, 50, 13272;
- 12bE. Y.-H. Hong, H.-L. Wong, V. W.-W. Yam, Chem. Eur. J. 2015, 21, 5732;
- 12cE. Y.-H. Hong, V. W.-W. Yam, ACS Appl. Mater. Interfaces 2017, 9, 2616.
- 13
- 13aH. Schmidbaur, H. G. Raubenheimer, L. Dobrzanska, Chem. Soc. Rev. 2014, 43, 345.
- 14
- 14aS. S.-Y. Chui, R. Chen, C.-M. Che, Angew. Chem. Int. Ed. 2006, 45, 1621; Angew. Chem. 2006, 118, 1651;
- 14bP. J. Barnard, L. E. Wedlock, M. V. Baker, S. J. Berners-Price, D. A. Joyce, B. W. Skelton, J. H. Steer, Angew. Chem. Int. Ed. 2006, 45, 5966; Angew. Chem. 2006, 118, 6112.
- 15T. E. Müller, S. W.-K. Choi, D. M. P. Mingos, D. Murphy, D. J. Williams, V. W.-W. Yam, J. Organomet. Chem. 1994, 484, 209.
- 16
- 16aH. Ito, T. Saito, N. Oshima, N. Kitamura, S. Ishizaka, Y. Hinatsu, M. Wakeshima, M. Kato, K. Tsuge, M. Sawamura, J. Am. Chem. Soc. 2008, 130, 10044;
- 16bT. Seki, N. Tokodai, S. Omagrai, T. Nakanish, Y. Hasegawa, T. Iwsa, T. Taketsugu, H. Ito, J. Am. Chem. Soc. 2017, 139, 6514;
- 16cM. Jin, T. Seki, H. Ito, J. Am. Chem. Soc. 2017, 139, 7452.
- 17
- 17aY. Sagara, T. Kato, Nat. Chem. 2009, 1, 605;
- 17bY. Sagara, S. Yamane, M. Mitani, C. Weder, T. Kato, Adv. Mater. 2016, 28, 1073;
- 17cB. Y.-W. Wong, H.-L. Wong, Y.-C. Wong, V. K.-M. Au, M.-Y. Chan, V. W.-W. Yam, Chem. Sci. 2017, 8, 6936.
- 18
- 18aV. W.-W. Yam, C.-C. Ko, N. Zhu, J. Am. Chem. Soc. 2004, 126, 12734;
- 18bP. H.-M. Lee, C.-C. Ko, N. Zhu, V. W.-W. Yam, J. Am. Chem. Soc. 2007, 129, 6058;
- 18cV. W.-W. Yam, J. K.-W. Lee, C.-C. Ko, N. Zhu, J. Am. Chem. Soc. 2009, 131, 912;
- 18dC.-T. Poon, W. H. Lam, H.-L. Wong, V. W.-W. Yam, J. Am. Chem. Soc. 2010, 132, 13992;
- 18eC.-T. Poon, W. H. Lam, H.-L. Wong, V. W.-W. Yam, J. Am. Chem. Soc. 2011, 133, 19622;
- 18fJ. C.-H. Chan, W. H. Lam, H.-L. Wong, N. Zhu, W.-T. Wong, V. W.-W. Yam, J. Am. Chem. Soc. 2011, 133, 12690;
- 18gJ. C.-H. Chan, W. H. Lam, V. W.-W. Yam, J. Am. Chem. Soc. 2014, 136, 16994.
- 19
- 19aA. S. D. Sandanayaka, K. Matsukawa, T. Ishi-i, S. Mataka, Y. Araki, O. Ito, J. Phys. Chem. B 2004, 108, 19995;
- 19bK. M. Omer, S.-Y. Ku, J.-Z. Cheng, S.-H. Chou, K.-T. Wong, A. J. Bard, J. Am. Chem. Soc. 2011, 133, 5492.
- 20T. Kudemac, T. Kobayashi, A. Uyama, K. Uchida, S. Nakamura, B. L. Feringa, J. Phys. Chem. A 2013, 117, 8222.