Chemoselective Synthesis of Z-Olefins through Rh-Catalyzed Formate-Mediated 1,6-Reduction
Raphael Dada
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
These authors contributed equally to this work.
Search for more papers by this authorZhongyu Wei
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
These authors contributed equally to this work.
Search for more papers by this authorRuohua Gui
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorCorresponding Author
Prof. Rylan J. Lundgren
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorRaphael Dada
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
These authors contributed equally to this work.
Search for more papers by this authorZhongyu Wei
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
These authors contributed equally to this work.
Search for more papers by this authorRuohua Gui
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorCorresponding Author
Prof. Rylan J. Lundgren
Department of Chemistry, University of Alberta, Edmonton, Alberta, T6G 2G2 Canada
Search for more papers by this authorGraphical Abstract
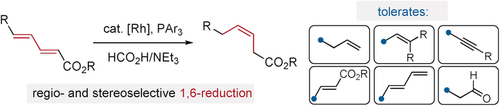
Two become one: Many prevailing methods for cis-olefination are complicated by the presence of multiple unsaturated units or electrophilic functional groups. In this study, Z-olefins are delivered through selective reduction of activated dienes using formic acid. The reaction proceeds with high regio- and stereoselectivity (typically >90:10 and >95:5, respectively) and preserves other alkenyl, alkynyl, protic, and electrophilic groups.
Abstract
Z-olefins are important functional units in synthetic chemistry; their preparation has thus received considerable attention. Many prevailing methods for cis-olefination are complicated by the presence of multiple unsaturated units or electrophilic functional groups. In this study, Z-olefins are delivered through selective reduction of activated dienes using formic acid. The reaction proceeds with high regio- and stereoselectivity (typically >90:10 and >95:5, respectively) and preserves other alkenyl, alkynyl, protic, and electrophilic groups.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201800361-sup-0001-misc_information.pdf17.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. Y. Siau, Y. Zhang, Y. Zhao in Stereoselective Alkene Synthesis, Vol. 327 (Ed.: ), 2012, p. 33;
- 1bC. Oger, L. Balas, T. Durand, J. M. Galano, Chem. Rev. 2013, 113, 1313;
- 1cM. B. Herbert, R. H. Grubbs, Angew. Chem. Int. Ed. 2015, 54, 5018; Angew. Chem. 2015, 127, 5104;
- 1dA. H. Hoveyda, R. K. M. Khan, S. Torker, S. J. Malcolmson in Handbook of Metathesis Vol. 2: Applications in Organic Synthesis Second Edition, Vol. 2 (Eds.: ), Wiley-VCH, Weinheim, 2015, pp. 503.
- 2T. Takeda, Modern Carbonyl Olefination, Wiley-VCH, Weinheim, 2004.
10.1002/3527601880 Google Scholar
- 3Y. Gu, S.-K. Tian in Stereoselective Alkene Synthesis (Ed.: ), Springer Berlin Heidelberg, Berlin, 2012, p. 197.
10.1007/128_2012_314 Google Scholar
- 4Recent examples:
- 4aE. D. Slack, C. M. Gabriel, B. H. Lipshutz, Angew. Chem. Int. Ed. 2014, 53, 14051; Angew. Chem. 2014, 126, 14275;
- 4bA. Fedorov, H. J. Liu, H. K. Lo, C. Coperet, J. Am. Chem. Soc. 2016, 138, 16502;
- 4cS. M. Fu, N. Y. Chen, X. F. Liu, Z. H. Shao, S. P. Luo, Q. Liu, J. Am. Chem. Soc. 2016, 138, 8588;
- 4dC. Y. Chen, Y. Huang, Z. P. Zhang, X. Q. Dong, X. M. Zhang, Chem. Commun. 2017, 53, 4612;
- 4eA. M. Whittaker, G. Lalic, Org. Lett. 2013, 15, 1112.
- 5Selected recent examples:
- 5aM. J. Koh, R. K. M. Khan, S. Torker, M. Yu, M. S. Mikus, A. H. Hoveyda, Nature 2015, 517, 181;
- 5bM. J. Koh, T. T. Nguyen, H. M. Zhang, R. R. Schrock, A. H. Hoveyda, Nature 2016, 531, 459;
- 5cS. X. Luo, J. S. Cannon, B. L. H. Taylor, K. M. Engle, K. N. Houk, R. H. Grubbs, J. Am. Chem. Soc. 2016, 138, 14039;
- 5dM. J. Koh, T. T. Nguyen, J. K. Lam, S. Torker, J. Hyvl, R. R. Schrock, A. H. Hoveyda, Nature 2017, 542, 80.
- 6Alkyne Z-carbofunctionalization:
- 6aC. W. Cheung, F. E. Zhurkin, X. L. Hu, J. Am. Chem. Soc. 2015, 137, 4932;
- 6bH. P. Deng, X. Z. Fan, Z. H. Chen, Q. H. Xu, J. J. Wu, J. Am. Chem. Soc. 2017, 139, 13579.
- 7
- 7aA. G. Csákÿ, G. de la Herran, M. C. Murcia, Chem. Soc. Rev. 2010, 39, 4080;
- 7bE. M. P. Silva, A. M. S. Silva, Synthesis 2012, 44, 3109;
- 7cT. E. Schmid, S. Drissi-Amraoui, C. Crevisy, O. Basle, M. Mauduit, Beilstein J. Org. Chem. 2015, 11, 2418;
- 7dP. Chauhan, U. Kaya, D. Enders, Adv. Synth. Catal. 2017, 359, 888;
- 7eT. Nishimura, Y. Yasuhara, T. Hayashi, Angew. Chem. Int. Ed. 2006, 45, 5164.
- 8
- 8aD. Uraguchi, K. Yoshioka, Y. Ueki, T. Ooi, J. Am. Chem. Soc. 2012, 134, 19370;
- 8bM. Tissot, A. Alexakis, Chem. Eur. J. 2013, 19, 11352;
- 8cZ. Wang, T. F. Kang, Q. Yao, J. Ji, X. H. Liu, L. L. Lin, X. M. Feng, Chem. Eur. J. 2015, 21, 7709;
- 8dF. K. Meng, X. B. Li, S. Torker, Y. Shi, X. Shen, A. H. Hoveyda, Nature 2016, 537, 387;
- 8eD. Uraguchi, K. Yoshioka, T. Ooi, Nat. Commun. 2017, 8, 10.
- 9
- 9aG. de la Herrán, C. Murcia, A. G. Csaky, Org. Lett. 2005, 7, 5629;
- 9bS. Okada, K. Arayama, R. Murayama, T. Ishizuka, K. Hara, N. Hirone, T. Hata, H. Urabe, Angew. Chem. Int. Ed. 2008, 47, 6860; Angew. Chem. 2008, 120, 6966;
- 9cK. S. Lee, A. H. Hoveyda, J. Am. Chem. Soc. 2010, 132, 2898;
- 9dK. S. Lee, H. Wu, F. Haeffner, A. H. Hoveyda, Organometallics 2012, 31, 7823.
- 10
- 10aM. Sodeoka, M. Shibasaki, Synthesis 1993, 643;
- 10bA. A. Vasil'ev, A. L. Vlasyuk, G. D. Gamalevich, E. P. Serebryakov, Bioorg. Med. Chem. 1996, 4, 389.
- 11
- 11aS. Steines, U. Englert, B. Driessen-Holscher, Chem. Commun. 2000, 217;
- 11bM. Kotova, E. Vyskocilova, L. Cerveny, Catal. Lett. 2017, 147, 1665.
- 12Recent examples of E-selective, Rh-catalyzed diene hydrofunctionalizations:
- 12aM. J. Goldfogel, S. J. Meek, Chem. Sci. 2016, 7, 4079;
- 12bX. H. Yang, V. M. Dong, J. Am. Chem. Soc. 2017, 139, 1774;
- 12cJ. S. Marcum, C. C. Roberts, R. S. Manan, T. N. Cervarich, S. J. Meek, J. Am. Chem. Soc. 2017, 139, 15580; Z-selective hydrofunctionalizations of simple 1,3-dienes: (hydroboration)
- 12dR. J. Ely, J. P. Morken, J. Am. Chem. Soc. 2010, 132, 2534; (hydrovinylation)
- 12eS. M. Jing, V. Balasanthiran, J. C. Gallucci, T. V. RajanBabu, J. Am. Chem. Soc. 2017, 139, 18034; (hydroxymethylation);
- 12fY. Y. Gui, N. Hu, X. W. Chen, L. Liao, T. Ju, J. H. Ye, Z. Zhang, J. Li, D. G. Yu, J. Am. Chem. Soc. 2017, 139, 17011; Examples of the use of formic acid in Rh-catalysis:
- 12gX. Wu, X. Li, A. Zanotti-Gerosa, A. Pettman, J. Liu, A. J. Mills, J. Xiao, Chem. Eur. J. 2008, 14, 2209;
- 12hY. J. Jang, E. M. Larin, M. Lautens, Angew. Chem. Int. Ed. 2017, 56, 11927; Angew. Chem. 2017, 129, 12089;
- 12iRh-catalyzed, Z-selective alkene isomerization:L. G. Zhou, Z. K. Yao, Z. X. Yu, Org. Lett. 2013, 15, 4634
- 131) The remaining mass balance is typically 5–10 % α,β-unsaturated ester; see the Supporting Information for the complete product distribution of the standard reaction and examples of other phosphines examined. The use of silane, borane, or H2 reducing agents resulted in poor yields of the 1,6 addition product. 2) See the Supporting Information for amide optimization data and examples of less successful substrates. 3) Rationalization for incomplete D incorporation could involve Rh-D/H exchange with non-formic acid hydrogens; D incorporation at other sites was not detected. 4) Formation of off-cycle Rh(dienoate)2+ species could explain the observed negative order in substrate; excess phosphine may be beneficial in formation of Rh(PPh3)(dienoate)+ from such intermediates.
- 14K. D. Collins, F. Glorius, Nat. Chem. 2013, 5, 597.
- 15A Rh-catalyzed process that generates Z-olefins from a proposed Rh-H intermediate: J. I. Martínez, J. J. Smith, H. B. Hepburn, H. W. Lam, Angew. Chem. Int. Ed. 2016, 55, 1108; Angew. Chem. 2016, 128, 1120.
- 16
- 16aA Rh-catalyzed reductive coupling reaction of aldehydes with dienes using BEt3 that, in a single example, gives a Z-addition product: M. Kimura, D. Nojiri, M. Fukushima, S. Oi, Y. Sonoda, Y. Inoue, Org. Lett. 2009, 11, 3794;
- 16bRh-catalyzed codimerization of olefins can give Z-addition products. A pioneering example: T. Alderson, E. L. Jenner, R. V. Lindsey, Jr., J. Am. Chem. Soc. 1965, 87, 5638.