Solvent-Dependent Asymmetric Synthesis of Alkynyl and Monofluoroalkenyl Isoindolinones by CpRhIII-Catalyzed C−H Activation
Graphical Abstract
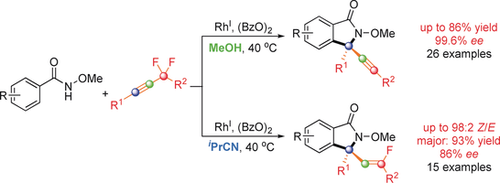
A matter of solvent: Alkynyl and monofluoroalkenyl isoindolinones were generated with good enantioselectivities from N-methoxy benzamides and α,α-difluoromethylene alkynes by C−H activation with a chiral CpRhIII catalyst. Remarkably, the product formation is solvent-dependent; whereas alkynyl isoindolinones are formed in methanol, monofluoroalkenyl isoindolinones are generated in isobutyronitrile.
Abstract
The asymmetric synthesis of alkynyl and monofluoroalkenyl isoindolinones from N-methoxy benzamides and α,α-difluoromethylene alkynes is enabled by C−H activation with a chiral CpRhIII catalyst. Remarkably, product formation is solvent-dependent; alkynyl isoindolinones are afforded in MeOH (up to 86 % yield, 99.6 % ee) whereas monofluoroalkenyl isoindolinones are generated in iPrCN (up to 98:2 Z/E, 93 % yield, 86 % ee). Mechanistic studies revealed chiral allene and E-configured alkenyl rhodium species as reaction intermediates. The latter is transformed into the corresponding Z-configured monofluoroalkene upon protonation in the iPrCN system and into an alkyne by an unusual anti β-F elimination in the MeOH system. Notably, kinetic resolution processes occur in this reaction. Despite the moderate enantiocontrol for the formation of the chiral allene, the Z-monofluoroalkenyl isoindolinones and alkynyl isoindolinones were obtained in good enantiopurities by one or two sequential kinetic resolution processes.