Organocatalytic Enantioselective Protonation for Photoreduction of Activated Ketones and Ketimines Induced by Visible Light
Lu Lin
Key Laboratory of Natural Medicine and Immuno-Engineering of Henan Province, Henan University, Jinming Campus, Kaifeng, Henan, 475004 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorXiangbin Bai
Key Laboratory of Natural Medicine and Immuno-Engineering of Henan Province, Henan University, Jinming Campus, Kaifeng, Henan, 475004 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorXinyi Ye
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, 637371 Singapore
Search for more papers by this authorXiaowei Zhao
Key Laboratory of Natural Medicine and Immuno-Engineering of Henan Province, Henan University, Jinming Campus, Kaifeng, Henan, 475004 P.R. China
Search for more papers by this authorProf. Dr. Choon-Hong Tan
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, 637371 Singapore
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhiyong Jiang
Key Laboratory of Natural Medicine and Immuno-Engineering of Henan Province, Henan University, Jinming Campus, Kaifeng, Henan, 475004 P.R. China
Search for more papers by this authorLu Lin
Key Laboratory of Natural Medicine and Immuno-Engineering of Henan Province, Henan University, Jinming Campus, Kaifeng, Henan, 475004 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorXiangbin Bai
Key Laboratory of Natural Medicine and Immuno-Engineering of Henan Province, Henan University, Jinming Campus, Kaifeng, Henan, 475004 P.R. China
These authors contributed equally to this work.
Search for more papers by this authorXinyi Ye
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, 637371 Singapore
Search for more papers by this authorXiaowei Zhao
Key Laboratory of Natural Medicine and Immuno-Engineering of Henan Province, Henan University, Jinming Campus, Kaifeng, Henan, 475004 P.R. China
Search for more papers by this authorProf. Dr. Choon-Hong Tan
Division of Chemistry and Biological Chemistry, Nanyang Technological University, 21 Nanyang Link, 637371 Singapore
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhiyong Jiang
Key Laboratory of Natural Medicine and Immuno-Engineering of Henan Province, Henan University, Jinming Campus, Kaifeng, Henan, 475004 P.R. China
Search for more papers by this authorGraphical Abstract
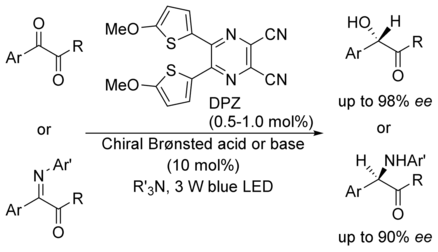
Enantioselective protonation: The first catalytic asymmetric photoreduction of 1,2-diketones and α-keto ketimines under visible light irradiation relies on a transition-metal-free cooperative catalysis platform that harnesses dicyanopyrazine-derived chromophore (DPZ) as the photoredox catalyst and a noncovalent chiral organocatalyst. A variety of chiral α-hydroxy ketones and α-amino ketones was obtained with high yields and enantioselectivities.
Abstract
The first catalytic asymmetric photoreduction of 1,2-diketones and α-keto ketimines under visible light irradiation is reported. A transition-metal-free synergistic catalysis platform harnessing dicyanopyrazine-derived chromophore (DPZ) as the photoredox catalyst and a non-covalent chiral organocatalyst is effective for these transformations. With the flexible use of a chiral Brønsted acid or base in H+ transfer interchange to control the elusive enantioselective protonation, a variety of chiral α-hydroxy ketones and α-amino ketones were obtained with high yields and enantioselectivities.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201707899-sup-0001-misc_information.pdf1.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322;
- 1bD. M. Schultz, T. P. Yoon, Science 2014, 343, 985;
- 1cJ. W. Beatty, C. R. J. Stephenson, Acc. Chem. Res. 2015, 48, 1474;
- 1dM. H. Shaw, J. Twilton, D. W. C. MacMillan, J. Org. Chem. 2016, 81, 6898;
- 1eN. A. Romero, D. A. Nicewicz, Chem. Rev. 2016, 116, 10075;
- 1fJ. Twilton, C. C. Le, P. Zhang, M. H. Shaw, R. W. Evans, D. W. C. MacMillan, Nat. Rev. Chem. 2017, 1, 0052.
- 2
- 2aO. Ishitani, C. Pac, H. Sakurai, J. Org. Chem. 1983, 48, 2941;
- 2bO. Ishitani, S. Yanagida, S. Takamuku, C. Pac, J. Org. Chem. 1987, 52, 2790;
- 2cI. Willner, T. Tsfania, Y. Eichen, J. Org. Chem. 1990, 55, 2656.
- 3
- 3aC. Wang, Z. Lu, Org. Chem. Front. 2015, 2, 179;
- 3bR. Brimioulle, D. Lenhart, M. M. Maturi, T. Bach, Angew. Chem. Int. Ed. 2015, 54, 3872; Angew. Chem. 2015, 127, 3944;
- 3cE. Meggers, Chem. Commun. 2015, 51, 3290;
- 3dS. Poplata, A. Tröster, Y.-Q. Zou, T. Bach, Chem. Rev. 2016, 116, 9748.
- 4
- 4aY. Ohgo, Y. Tashiro, S. Takeuchi, Bull. Chem. Soc. Jpn. 1987, 60, 1549;
- 4bL. De Luca, A. Mezzetti, Angew. Chem. Int. Ed. 2017, 56, 11949; Angew. Chem. 2017, 129, 12111.
- 5B. Procuranti, S. J. Connon, Chem. Commun. 2007, 1421.
- 6F. A. Davis, B.-C. Chen, Chem. Rev. 1992, 92, 919.
- 7J. T. Mohr, A. Y. Hong, B. M. Stoltz, Nat. Chem. 2009, 1, 359.
- 8L. J. Rono, H. G. Yayla, D. Y. Wang, M. F. Armstrong, R. R. Knowles, J. Am. Chem. Soc. 2013, 135, 17735.
- 9
- 9aB. Zhu, R. Lee, J. Li, X. Ye, S.-N. Hong, S. Qiu, M. L. Coote, Z. Jiang, Angew. Chem. Int. Ed. 2016, 55, 1299; Angew. Chem. 2016, 128, 1321;
- 9bS. Hong, Y. Liu, R. Lee, Z. Jiang, Chem. Commun. 2017, 53, 7493.
- 10
- 10aX. Liu, X. Ye, F. Bureš, H. Liu, Z. Jiang, Angew. Chem. Int. Ed. 2015, 54, 11443; Angew. Chem. 2015, 127, 11605;
- 10bG. Wei, C. Zhang, F. Bureš, X. Ye, C.-H. Tan, Z. Jiang, ACS Catal. 2016, 6, 3708;
- 10cC. Zhang, S. Li, F. Bureš, R. Lee, X. Ye, Z. Jiang, ACS Catal. 2016, 6, 6853.
- 11
- 11aA. E. Allen, D. W. C. MacMillan, Chem. Sci. 2012, 3, 633;
- 11bM. N. Hopkinson, B. Sahoo, J.-L. Li, F. Glorius, Chem. Eur. J. 2014, 20, 3874;
- 11cT. P. Yoon, Acc. Chem. Res. 2016, 49, 2307;
- 11dK. L. Skubi, T. R. Blum, T. P. Yoon, Chem. Rev. 2016, 116, 10035.
- 12
- 12aD. A. Nicewicz, D. W. C. MacMillan, Science 2008, 322, 77;
- 12bD. A. Nagib, M. E. Scott, D. W. C. MacMillan, J. Am. Chem. Soc. 2009, 131, 10875;
- 12cM. Neumann, S. Füldner, B. König, K. Zeitler, Angew. Chem. Int. Ed. 2011, 50, 951; Angew. Chem. 2011, 123, 981;
- 12dD. A. DiRocco, T. Rovis, J. Am. Chem. Soc. 2012, 134, 8094;
- 12eM. Cherevatskaya, M. Neumann, S. Füldner, C. Harlander, S. Kümmel, S. Dankesreiter, A. Pfitzner, K. Zeitler, B. König, Angew. Chem. Int. Ed. 2012, 51, 4062; Angew. Chem. 2012, 124, 4138;
- 12fM. T. Pirnot, D. A. Rankic, D. B. C. Martin, D. W. C. MacMillan, Science 2013, 339, 1593;
- 12gJ. Du, K. L. Skubi, D. M. Schultz, T. P. Yoon, Science 2014, 344, 392;
- 12hG. Bergonzini, C. S. Schindler, C.-J. Wallentin, E. N. Jacobsen, C. R. J. Stephenson, Chem. Sci. 2014, 5, 112;
- 12iY. Zhu, L. Zhang, S. Luo, J. Am. Chem. Soc. 2014, 136, 14642;
- 12jO. Gutierrez, J. C. Tellis, D. N. Primer, G. A. Molander, M. C. Kozlowski, J. Am. Chem. Soc. 2015, 137, 4896;
- 12kL. Ruiz Espelt, I. S. McPherson, E. M. Wiensch, T. P. Yoon, J. Am. Chem. Soc. 2015, 137, 2452;
- 12lD. Uraguchi, N. Kinoshita, T. Kizu, T. Ooi, J. Am. Chem. Soc. 2015, 137, 13768;
- 12mT. Kizu, D. Uraguchi, T. Ooi, J. Org. Chem. 2016, 81, 6953;
- 12nZ. Zuo, H. Cong, W. Li, J. Choi, G. C. Fu, D. W. C. MacMillan, J. Am. Chem. Soc. 2016, 138, 1832;
- 12oA. G. Amador, E. M. Sherbrook, T. P. Yoon, J. Am. Chem. Soc. 2016, 138, 4722;
- 12pC. Wang, K. Harms, E. Meggers, Angew. Chem. Int. Ed. 2016, 55, 13495; Angew. Chem. 2016, 128, 13693;
- 12qX. Huang, R. D. Webster, K. Harms, E. Meggers, J. Am. Chem. Soc. 2016, 138, 12636;
- 12rJ. J. Murphy, D. Bastida, S. Paria, M. Fagnoni, P. Melchiorre, Nature 2016, 532, 218;
- 12sQ. Yang, L. Zhang, C. Ye, S. Luo, L.-Z. Wu, C.-H. Tung, Angew. Chem. Int. Ed. 2017, 56, 3694; Angew. Chem. 2017, 129, 3748.
- 13
- 13aT. Bach, H. Bergmann, B. Grosch, K. Harms, J. Am. Chem. Soc. 2002, 124, 7982;
- 13bA. Bauer, F. Westkämper, S. Grimme, T. Bach, Nature 2005, 436, 1139;
- 13cR. Brimioulle, T. Bach, Science 2013, 342, 840;
- 13dH. Huo, X. Shen, C. Wang, L. Zhang, P. Röse, L.-A. Chen, K. Harms, M. Marsch, G. Hilt, E. Meggers, Nature 2014, 515, 100;
- 13eH. Huo, C. Wang, K. Harms, E. Meggers, J. Am. Chem. Soc. 2015, 137, 9551;
- 13fR. Brimioulle, A. Bauer, T. Bach, J. Am. Chem. Soc. 2015, 137, 5170;
- 13gA. Tröster, R. Alonso, A. Bauer, T. Bach, J. Am. Chem. Soc. 2016, 138, 7808;
- 13hW. Ding, L.-Q. Lu, Q.-Q. Zhou, Y. Wei, J.-R. Chen, W.-J. Xiao, J. Am. Chem. Soc. 2017, 139, 63;
- 13iX. Huang, T. R. Quinn, K. Harms, R. D. Webster, L. Zhang, O. Wiest, E. Meggers, J. Am. Chem. Soc. 2017, 139, 9120.
- 14DPZ: E t(S*/S.−)=+0.91 V vs. SCE in CH2Cl2,
 −1.45 V vs. SCE in CH2Cl2 for DPZ.−. Ru(bpy)32+:
−1.45 V vs. SCE in CH2Cl2 for DPZ.−. Ru(bpy)32+:  +0.77 V vs. SCE in CH3CN.
+0.77 V vs. SCE in CH3CN.
- 15 Topics in Current Chemistry, Vol. 291 (Ed.: ), Springer, Berlin, 2010.
- 16X. Fu, W.-T. Luo, Y. Zhang, T. Chen, T. Ma, H. Liu, J. Wang, C−H. Tan, Angew. Chem. Int. Ed. 2009, 48, 7387; Angew. Chem. 2009, 121, 7523.
- 17See the Supporting Information for details.
- 18C.-Y. Kuo, M.-J. Wu, C.-C. Lin, Eur. J. Med. Chem. 2010, 45, 55.
- 19
- 19aC. G. S. Lima, T. de M. Lima, M. Duarte, I. D. Jurberg, Paixão, ACS Catal. 2016, 6, 1389;
- 19bE. Arceo, I. D. Jurberg, A. Alvarez-Fernandez, P. Melchiorre, Nat. Chem. 2013, 5, 750;
- 19cM. Nappi, G. Bergonzini, P. Melchiorre, Angew. Chem. Int. Ed. 2014, 53, 4921; Angew. Chem. 2014, 126, 5021;
- 19dM. Silvi, E. Arceo, I. D. Jurberg, C. Cassani, P. Melchiorre, J. Am. Chem. Soc. 2015, 137, 6120;
- 19eA. Bahamonde, P. Melchiorre, J. Am. Chem. Soc. 2016, 138, 8019;
- 19fY.-Y. Liu, X.-Y. Yu, J.-R. Chen, M.-M. Qiao, X. Qi, D.-Q. Shi, W.-J. Xiao, Angew. Chem. Int. Ed. 2017, 56, 9527; Angew. Chem. 2017, 129, 9655;
- 19gM. Silvi, C. Verrier, Y. P. Rey, L. Buzzetti, P. Melchiorre, Nat. Chem. 2017, 9, 868.
- 20C. Bouteiller, J. Becerril-Ortega, P. Marchand, O. Nicole, L. Barre, A. Buisson, C. Perrio, Org. Biomol. Chem. 2010, 8, 1111.
- 21W. Wen, Y. Zeng, L.-Y. Peng, L.-N. Fu, Q.-X. Guo, Org. Lett. 2015, 17, 3922.
- 22No reaction was found between THIQ-2 and C3.
- 23L. Ruiz Espelt, E. M. Wiensch, T. P. Yoon, J. Org. Chem. 2013, 78, 4107.
- 24Since ET (DPZ)=46.4 kcal mol−1 [10c] and ET (benzil)=54.4 kcal mol−1 (see: X.-H. Duan, R.-X. He, X.-Y. Li, C.-Y. Luo, Chem. J. Chin. Univ. 2005, 26, 1686), energy transfer might be excluded.
- 25No racemization was observed when 2 a (91 % ee) was in the presence of 2.0 equiv of THIQ-2, Et3N or iPr2EtN in CH2Cl2 at 25 °C for 12 h.