The Tris(pentafluoroethyl)silanide Anion
Nico Schwarze
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorDr. Simon Steinhauer
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorBeate Neumann
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorDr. Hans-Georg Stammler
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Berthold Hoge
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorNico Schwarze
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorDr. Simon Steinhauer
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorBeate Neumann
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorDr. Hans-Georg Stammler
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorCorresponding Author
Prof. Dr. Berthold Hoge
Universität Bielefeld, Fakultät für Chemie, Centrum für Molekulare Materialien, Universitätsstrasse 25, 33615 Bielefeld, Germany
Search for more papers by this authorGraphical Abstract
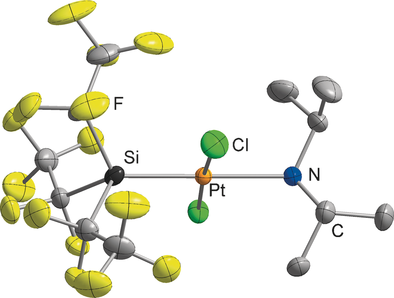
Tris(pentafluoroethyl)silane was used for hydrosilylation reactions. It can be deprotonated by sterically demanding bases at low temperatures, which leads to the formation of the corresponding silanide ion. Addition of crown ethers or cryptands allows the isolation and characterization of the salt-like tris(pentafluoroethyl)silanide at room temperature. Interception of the silanide with PtIICl2 resulted in the formation of a silyl-substituted platinum complex.
Abstract
Tris(pentafluoroethyl)silane, which is conveniently accessible by the treatment of Si(C2F5)3X (X=Cl, Br) with Bu3SnH, was successfully employed for hydrosilylation reactions. In the presence of a palladium catalyst, hydrosilylation of phenylacetylene with Si(C2F5)3H affords the product of an α-addition whereas the reaction of trimethylsilylacetylene with the silane leads to the β-trans product. Tris(pentafluoroethyl)silane can be deprotonated by sterically demanding bases such as lithium diisopropylamide at low temperatures to give the corresponding silanide ion. The addition of crown ethers or cryptands to this highly reactive species enabled the isolation and characterization of salt-like tris(pentafluoroethyl)silanide at room temperature.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201609096-sup-0001-misc_information.pdf201.9 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. Scheschkewitz, C. Präsang, Struct. Bonding (Berlin) 2014, 156, 1–48.
- 2C. A. Kraus, H. Eatough, J. Am. Chem. Soc. 1933, 55, 5008–5014.
- 3C. A. Kraus, W. K. Nelson, J. Am. Chem. Soc. 1934, 56, 195–202.
- 4H. Gilman, T. C. Wu, J. Am. Chem. Soc. 1951, 73, 4031–4033.
- 5R. A. Benkeser, R. G. Severson, J. Am. Chem. Soc. 1951, 73, 1424–1427.
- 6H.-W. Lerner, Coord. Chem. Rev. 2005, 249, 781–798.
- 7A. Lorbach, S. Breitung, I. Sänger, F. Schödel, M. Bolte, M. Wagner, H.-W. Lerner, Inorg. Chim. Acta 2011, 378, 1–9.
- 8K. Tamao, A. Kawachi, Y. Ito, J. Am. Chem. Soc. 1992, 114, 3989–3990.
- 9
- 9aS. Nozakura, S. Konotsune, Bull. Chem. Soc. Jpn. 1956, 29, 322–326;
- 9bR. A. Benkeser, Acc. Chem. Res. 1971, 4, 94–100;
- 9cG. S. Li, D. F. Ehler, R. A. Benkeser, Organic Syntheses, Collect. Vol VI, Wiley, New York, 1988, p. 747;
- 9dJ. Tillmann, L. Meyer, J. I. Schweizer, M. Bolte, H.-W. Lerner, M. Wagner, M. C. Holthausen, Chem. Eur. J. 2014, 20, 9234–9239;
- 9eJ. Tillmann, J. H. Wender, U. Bahr, M. Bolte, H.-W. Lerner, M. C. Holthausen, M. Wagner, Angew. Chem. Int. Ed. 2015, 54, 5429–5433; Angew. Chem. 2015, 127, 5519–5523.
- 10H. Watanabe, K. Higuchi, T. Goto, T. Muraoka, J. Inose, M. Kageyama, Y. Lizuka, M. Nozaki, Y. Nagai, J. Organomet. Chem. 1981, 218, 27–39.
- 11C. Krempner, M. H. Chisholm, J. Gallucci, Angew. Chem. Int. Ed. 2008, 47, 410–413; Angew. Chem. 2008, 120, 416–420.
- 12K. W. Klinkhammer, Chem. Eur. J. 1997, 3, 1418–1431.
- 13V. D. Thalangamaarachchige, D. K. Unruh, D. B. Cordes, C. Krempner, Inorg. Chem. 2015, 54, 4189–4191.
- 14G. Becker, H.-M. Hartmann, A. Münch, H. Riffel, Z. Anorg. Allg. Chem. 1985, 530, 29–42.
- 15S. Steinhauer, J. Bader, H.-G. Stammler, N. Ignat'ev, B. Hoge, Angew. Chem. Int. Ed. 2014, 53, 5206–5209; Angew. Chem. 2014, 126, 5307–5310.
- 16
- 16aS. Steinhauer, H.-G. Stammler, B. Neumann, N. Ignat'ev, B. Hoge, Angew. Chem. Int. Ed. 2014, 53, 562–564; Angew. Chem. 2014, 126, 573–575;
- 16bN. Ignat'ev, M. Schulte, B. Hoge, S. Steinhauer (Merck Patent GmbH), DE 102012006896 A1, 2012;
- 16cB. Hoge, N. Ignat'ev, M. Schulte, S. Steinhauer (BASF SE), WO 2013\150448 A1, 2013;
- 16dS. Steinhauer, T. Böttcher, N. Schwarze, B. Neumann, H.-G. Stammler, B. Hoge, Angew. Chem. Int. Ed. 2014, 53, 13269–13272; Angew. Chem. 2014, 126, 13485–13488.
- 17
- 17aB. Waerder, S. Steinhauer, B. Neumann, G. Stammler, A. Mix, Y. V. Vishnevskiy, B. Hoge, N. Mitzel, Angew. Chem. Int. Ed. 2014, 53, 11640–11644; Angew. Chem. 2014, 126, 11824–11828;
- 17bM. Heinrich, A. Marhold, A. Kolomeitsev, A. Kadyrov, G.-V. Röschenthaler, J. Barten, DE10128703A1, 2001;
- 17cM. H. Königsmann, PhD Thesis, Universität Bremen, 2005.
- 18B. Waerder, S. Steinhauer, J. Bader, B. Neumann, H.-G. Stammler, Y. V. Vishnevskiy, B. Hoge, N. W. Mitzel, Dalton Trans. 2015, 44, 13347.
- 19J. Ackermann, V. Damrath, Chem. Unserer Zeit 1989, 23, 86–99.
- 20R. A. Benkeser, K. M. Voley, J. B. Grutzner, W. E. Smith, J. Am. Chem. Soc. 1970, 92, 697–698.
- 21J. L. Duncan, J. L. Harvie, D. C. McKean, S. Cradock, J. Mol. Struct. 1986, 145, 225–242.
- 22
- 22aY. Kawanami, K. Yamamoto, Synlett 1995, 1232–1234;
- 22bT. Shimada, K. Mukaide, A. Shinohara, J. W. Han, T. Hayashi, J. Am. Chem. Soc. 2002, 124, 1584–1585.
- 23
- 23aH. Schmidbaur, J. Ebenhöch, Z. Naturforsch. B 1987, 42, 1543–1548;
- 23bM. Chauhan, B. J. Hauck, L. P. Keller, P. Boudjouk, J. Organomet. Chem. 2002, 645, 1–13.
- 24T. Hayashi in Comprehensive asymmetric catalysis (Eds.: ), Springer, London, 2003, pp. 319–333.
- 25G. K. S. Prakash, P. V. Jog, P. T. D. Batamack, G. A. Olah, Science 2012, 338, 1324–1327.
- 26G. K. S. Prakash, F. Wang, Z. Zhang, R. Haiges, M. Rahm, K. O. Christe, T. Mathew, G. A. Olah, Angew. Chem. Int. Ed. 2014, 53, 11575–11578; Angew. Chem. 2014, 126, 11759–11762.
- 27A. Dawn, K. S. Andrew, D. S. Yufit, Y. Hong, J. Prakasha Reddy, C. D. Jones, J. A. Aguilar, J. W. Steed, Cryst. Growth Des. 2015, 15, 4591–4599.
- 28
- 28aF. Ozawa, J. Kamite, Organometallics 1998, 17, 5630–5639;
- 28bY.-J. Kim, E.-H. Choi, S. W. Lee, Organometallics 2003, 22, 3316–3319.
- 29O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Crystallogr. 2009, 42, 339–341.
- 30G. M. Sheldrick, Acta Crystallogr. Sect. A 2008, 64, 112–122.