Fusing Porphyrins and Phospholes: Synthesis and Analysis of a Phosphorus-Containing Porphyrin
Corresponding Author
Dr. Tomohiro Higashino
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorTomoki Yamada
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorDr. Tsuneaki Sakurai
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorProf. Dr. Shu Seki
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Hiroshi Imahori
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Institute for Integrated Cell Material Sciences (WPI-iCeMS), Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Dr. Tomohiro Higashino
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorTomoki Yamada
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorDr. Tsuneaki Sakurai
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorProf. Dr. Shu Seki
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Hiroshi Imahori
Department of Molecular Engineering, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Institute for Integrated Cell Material Sciences (WPI-iCeMS), Kyoto University, Nishikyo-ku, Kyoto, 615-8510 Japan
Search for more papers by this authorGraphical Abstract
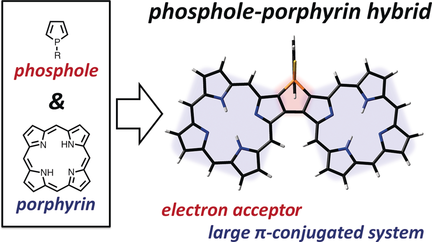
A phosphole-fused porphyrin dimer as a representative of a new class of phosphorus-containing porphyrins was synthesized. This structure exhibits remarkably broadened absorption as well as unique optoelectronic properties and is a good electron acceptor owing to the unique phosphole-fused structure.
Abstract
A phosphole-fused porphyrin dimer, as a representative of a new class of porphyrins with a phosphorus atom, was synthesized for the first time. The porphyrin dimer exhibits remarkably broadened absorption, indicating effective π-conjugation over the two porphyrins through the phosphole moiety. The porphyrin dimer possesses excellent electron-accepting character, which is comparable to that of a representative electron-accepting material, [60]PCBM. These results provide access to a new class of phosphorus-containing porphyrins with unique optoelectronic properties.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201607417-sup-0001-misc_information.pdf2.7 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a The Porphyrin Handbook, Vol. 1–10 (Eds.: ), Academic Press, San Diego, 2000;
- 1bD. Gust, T. A. Moore, A. L. Moore, Acc. Chem. Res. 2001, 34, 40–48;
- 1cD. Holten, D. F. Bocian, J. S. Lindsey, Acc. Chem. Res. 2002, 35, 57–69;
- 1dH. Imahori, J. Phys. Chem. B 2004, 108, 6130–6143;
- 1eM. Ethirajan, Y. Chen, P. Joshi, R. K. Pandey, Chem. Soc. Rev. 2011, 40, 340–362;
- 1fT. Tanaka, A. Osuka, Chem. Soc. Rev. 2015, 44, 943–969;
- 1gT. Higashino, H. Imahori, Dalton Trans. 2015, 44, 448–463.
- 2
- 2aB. M. J. M. Suijkerbuijk, R. J. M. Klein Gebbink, Angew. Chem. Int. Ed. 2008, 47, 7396–7421; Angew. Chem. 2008, 120, 7506–7532;
- 2bM. O. Senge, Chem. Commun. 2011, 47, 1943–1960;
- 2cH. Yorimitsu, A. Osuka, Asian J. Org. Chem. 2013, 2, 356–373.
- 3
- 3aP. J. Chmielewski, L. Latos-Grażyński, Coord. Chem. Rev. 2005, 249, 2510–2533;
- 3bI. Gupta, M. Ravikanth, Coord. Chem. Rev. 2006, 250, 468–518.
- 4
- 4aH. Segawa, K. Kunimoto, K. Susumu, M. Taniguchi, T. Shimidzu, J. Am. Chem. Soc. 1994, 116, 11193–11194;
- 4bK. Susumu, K. Kunimoto, H. Segawa, T. Shimidzu, J. Phys. Chem. 1995, 99, 29–34.
- 5
- 5aA. Młodzianowska, L. Latos-Grażyński, L. Szterenberg, Inorg. Chem. 2008, 47, 6364–6374;
- 5bE. Pacholska-Dudziak, F. Ulatowski, Z. Ciunik, L. Latos-Grażyński, Chem. Eur. J. 2009, 15, 10924–10929.
- 6
- 6aY. Matano, K. Matsumoto, Y. Nakao, H. Uno, S. Sakaki, H. Imahori, J. Am. Chem. Soc. 2008, 130, 4588–4589;
- 6bY. Matano, K. Matsumoto, H. Hayashi, Y. Nakao, T. Kumpulainen, V. Chukharev, N. V. Tkachenko, H. Lemmetyinen, S. Shimizu, N. Kobayashi, D. Sakamaki, A. Ito, K. Tanaka, H. Imahori, J. Am. Chem. Soc. 2012, 134, 1825–1839;
- 6cK. Fujimoto, T. Yoneda, H. Yorimitsu, A. Osuka, Angew. Chem. Int. Ed. 2014, 53, 1127–1130; Angew. Chem. 2014, 126, 1145–1148.
- 7
- 7aY. Matano, H. Imahori, Acc. Chem. Res. 2009, 42, 1193–1204;
- 7bY. Matano, T. Nakabuchi, T. Miyajima, H. Imahori, H. Nakano, Org. Lett. 2006, 8, 5713–5716;
- 7cY. Matano, T. Nakabuchi, S. Fujishige, H. Nakano, H. Imahori, J. Am. Chem. Soc. 2008, 130, 16446–16447;
- 7dY. Matano, M. Nakashima, T. Nakabuchi, H. Imahori, S. Fujishige, H. Nakano, Org. Lett. 2008, 10, 553–556;
- 7eT. Nakabuchi, Y. Matano, H. Imahori, Org. Lett. 2010, 12, 1112–1115;
- 7fY. Matano, T. Nakabuchi, H. Imahori, Pure Appl. Chem. 2010, 82, 583–593;
- 7gT. Nakabuchi, M. Nakashima, S. Fujishige, H. Nakano, Y. Matano, H. Imahori, J. Org. Chem. 2010, 75, 375–389.
- 8
- 8aA. Fukazawa, S. Yamaguchi, Chem. Asian J. 2009, 4, 1386–1400;
- 8bY. Matano, H. Imahori, Org. Biomol. Chem. 2009, 7, 1258–1271;
- 8cY. Ren, T. Baumgartner, Dalton Trans. 2012, 41, 7792–7800;
- 8dT. Baumgartner, Acc. Chem. Res. 2014, 47, 1613–1622;
- 8eM. Stolar, T. Baumgartner, Chem. Asian J. 2014, 9, 1212–1225.
- 9T. Higashino, H. Imahori, Chem. Eur. J. 2015, 21, 13375–13381.
- 10Crystallographic data for 12: C74H53N8PS⋅C3H8O⋅0.5(C2H4Cl2), Mr=1226.84, monoclinic, C2/c (No.15), a=23.139(10), b=18.670(8), c=30.139(12) Å, β=90.068(11)°, V=13020(9) Å3, ρcalcd=1.252 g cm−3, Z=8, R1=0.0836 [I>2σ(I)], wR2=0.3069 (all data), GOF=1.067. CCDC 1495318 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 11
- 11aA. Wakamiya, H. Nishimura, T. Fukushima, F. Suzuki, A. Saeki, S. Seki, I. Osaka, T. Sasamori, M. Murata, Y. Murata, H. Kaji, Angew. Chem. Int. Ed. 2014, 53, 5800–5804; Angew. Chem. 2014, 126, 5910–5914;
- 11bW. Nakanishi, N. Matsuyama, D. Hara, A. Saeki, S. Hitosugi, S. Seki, H. Isobe, Chem. Asian J. 2014, 9, 1782–1785.
- 12C. Ryppa, M. O. Senge, S. S. Hatscher, E. Kleinpeter, P. Wacker, U. Schilde, A. Wiehe, Chem. Eur. J. 2005, 11, 3427–3442.
- 13
- 13a5,10,15,20-Tetra(4-tolyl)porphyrin (λQ=647 nm in CH2Cl2): S. Zakavi, S. Hoseini, RSC Adv. 2015, 5, 106774–106786;
- 13bβ-β′-bis[5,10,15,20-tetra(4-tolyl)porphyrin] (λQ=655 nm in CH2Cl2): G. Bringmann, D. C. G. Götz, T. A. M. Gulder, T. H. Gehrke, T. Bruhn, T. Kupfer, K. Radacki, H. Braunschweig, A. Heckmann, C. Lambert, J. Am. Chem. Soc. 2008, 130, 17812–17825.
- 14
- 14aY. Matano, T. Miyajima, T. Fukushima, H. Kaji, Y. Kimura, H. Imahori, Chem. Eur. J. 2008, 14, 8102–8115;
- 14bA. Saito, T. Miyajima, M. Nakashima, T. Fukushima, H. Kaji, Y. Matano, H. Imahori, Chem. Eur. J. 2009, 15, 10000–10004;
- 14cY. Matano, A. Saito, T. Fukushima, Y. Tokudome, F. Suzuki, D. Sakamaki, H. Kaji, A. Ito, K. Tanaka, H. Imahori, Angew. Chem. Int. Ed. 2011, 50, 8016–8020; Angew. Chem. 2011, 123, 8166–8170;
- 14dY. Dienes, M. Eggenstein, T. Kárpáti, T. C. Sutherland, L. Nyulászi, T. Baumgartner, Chem. Eur. J. 2008, 14, 9878–9889;
- 14eY. Ren, Y. Dienes, S. Hettel, M. Parvez, B. Hoge, T. Baumgartner, Organometallics 2009, 28, 734–740;
- 14fH. Tsuji, K. Sato, Y. Sato, E. Nakamura, J. Mater. Chem. 2009, 19, 3364–3366;
- 14gH. Tsuji, K. Sato, Y. Sato, E. Nakamura, Chem. Asian J. 2010, 5, 1294–1297;
- 14hP.-A. Bouit, A. Escande, R. Szűcs, D. Szieberth, C. Lescop, L. Nyulászi, M. Hissler, R. Réau, J. Am. Chem. Soc. 2012, 134, 6524–6527;
- 14iF. Riobé, R. Szűcs, P.-A. Bouit, D. Tondelier, B. Geffroy, F. Aparicio, J. Buendía, L. Sánchez, R. Réau, L. Nyulászi, M. Hissler, Chem. Eur. J. 2015, 21, 6547–6556.
- 15J. Pommerehne, H. Vestweber, W. Guss, R. F. Mahrt, H. Bässler, M. Porsch, J. Daub, Adv. Mater. 1995, 7, 551–554.
- 16
- 16aJ. C. Hummelen, B. W. Knight, F. LePeq, F. Wudl, J. Yao, C. L. Wilkins, J. Org. Chem. 1995, 60, 532–538;
- 16bR. Tao, T. Umeyama, T. Higashino, T. Koganezawa, H. Imahori, Chem. Commun. 2015, 51, 8233–8236.
- 17
- 17aY. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade, H. Yan, Nat. Commun. 2014, 5, 5293;
- 17bJ. E. Anthony, A. Facchetti, M. Heeney, S. R. Marder, X. Zhan, Adv. Mater. 2010, 22, 3876–3892;
- 17cJ. L. Delgado, P.-A. Bouit, S. Filippone, M. Herranz, N. Martín, Chem. Commun. 2010, 46, 4853–4865;
- 17dG. Li, R. Zhu, Y. Yang, Nat. Photonics 2012, 6, 153–161.
- 18
- 18aA. Saeki, Y. Koizumi, T. Aida, S. Seki, Acc. Chem. Res. 2012, 45, 1193–1202;
- 18bA. Saeki, S. Seki, T. Takenobu, Y. Iwasa, S. Tagawa, Adv. Mater. 2008, 20, 920–923;
- 18cH. Hayashi, W. Nihashi, T. Umeyama, Y. Matano, S. Seki, Y. Shimizu, H. Imahori, J. Am. Chem. Soc. 2011, 133, 10736–10739;
- 18dY. Matano, H. Ohkubo, Y. Honsho, A. Saito, S. Seki, H. Imahori, Org. Lett. 2013, 15, 932–935.