Rotationally Active Ligands: Dialing-Up the Co-conformations of a [2]Rotaxane for Metal Ion Binding
Dr. Giorgio Baggi
Department of Chemistry and Biochemistry, University of Windsor, Windsor, Ontario, N9B 3P4 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen J. Loeb
Department of Chemistry and Biochemistry, University of Windsor, Windsor, Ontario, N9B 3P4 Canada
Search for more papers by this authorDr. Giorgio Baggi
Department of Chemistry and Biochemistry, University of Windsor, Windsor, Ontario, N9B 3P4 Canada
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen J. Loeb
Department of Chemistry and Biochemistry, University of Windsor, Windsor, Ontario, N9B 3P4 Canada
Search for more papers by this authorGraphical Abstract
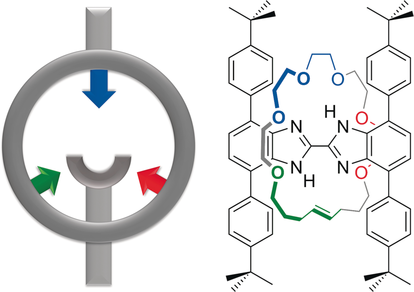
A rigid, H-shaped [2]rotaxane ligand with different sets of donor atoms on the axle and wheel components was prepared. The dynamics of the rotaxane and the orthogonal arrangement of the donors allows access to different co-conformations by simple rotation of the wheel about the axle. Three different rotational isomers were observed and structurally characterized for the neutral ligand and coordination complexes with Li+ and Cu+ ions.
Abstract
A novel [2]rotaxane was constructed that has a bidentate N,N′-chelate as part of a rigid, H-shaped axle and a 24-membered crown ether macrocycle containing six ether O-atoms and an olefinic group as the wheel. This unique topology produces a ligand with the ability to dial-up different donor sets for complexation to metal ions by simply rotating the wheel about the axle. The solution and solid-state structures of the free ligand and complexes with Li+ and Cu+ show how the ligand adopts different rotational co-conformations for each. The Li+ ion uses the N,N′-chelate and O-donors while the Cu+ center is coordinated to both O-donors and the olefinic group. This concept of rotationally active ligands should be possible with a wide variety of donor sets and could find broad application in areas of coordination chemistry, such as catalysis and metal sequestration.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201607281-sup-0001-misc_information.pdf3.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ.-P. Sauvage, Acc. Chem. Res. 1998, 31, 611–619;
- 1bT. J. Hubin, D. H. Busch, Coord. Chem. Rev. 2000, 200–202, 5–52;
- 1cJ.-P. Collin, C. Dietrich-Buchecker, P. Gaviña, M. C. Jimenez-Molero, J.-P. Sauvage, Acc. Chem. Res. 2001, 34, 477–487;
- 1dC. Dietrich-Buchecker, M. C. Jimenez-Molero, V. Sartor, J.-P. Sauvage, Pure Appl. Chem. 2003, 75, 1383–1393;
- 1eF. Aricó, J. D. Badjic, S. J. Cantrill, A. H. Flood, K. C.-F. Leung, Y. Liu, J. F. Stoddart, Top. Curr. Chem. 2005, 249, 203–259;
- 1fJ. F. Stoddart, Chem. Soc. Rev. 2009, 38, 1521–1529;
- 1gJ. D. Crowley, S. M. Goldup, A.-L. Lee, D. A. Leigh, R. T. McBurney, Chem. Soc. Rev. 2009, 38, 1530–1541;
- 1hJ. E. Beves, B. A. Blight, C. J. Campbell, D. A. Leigh, R. T. McBurney, Angew. Chem. Int. Ed. 2011, 50, 9260–9327; Angew. Chem. 2011, 123, 9428–9499;
- 1iS. F. M. van Dongen, S. Cantekin, J. A. A. W. Elemans, A. E. Rowan, R. J. M. Nolte, Chem. Soc. Rev. 2014, 43, 99–122;
- 1jG. Gil-Ramírez, D. A. Leigh, A. J. Stephens, Angew. Chem. Int. Ed. 2015, 54, 6110–6150; Angew. Chem. 2015, 127, 6208–6249.
- 2
- 2aC. O. Dietrich-Buchecker, J.-P. Sauvage, J.-P. Kintzinger, Tetrahedron Lett. 1983, 24, 5095–5098;
- 2bC. O. Dietrich-Buchecker, J.-P. Sauvage, J.-M. Kern, J. Am. Chem. Soc. 1984, 106, 3043–3045;
- 2cM. Cesario, C. O. Dietrich-Buchecker, J. Guilhem, C. Pascard, J.-P. Sauvage, J. Chem. Soc. Chem. Commun. 1985, 244–247;
- 2dA.-M. Albrecht-Gary, Z. Saad, C. O. Dietrich-Buchecker, J.-P. Sauvage, J. Am. Chem. Soc. 1985, 107, 3205–3209;
- 2eA.-M. Albrecht-Gary, C. Dietrich-Buchecker, Z. Saad, J.-P. Sauvage, J. Weiss, J. Chem. Soc. Chem. Commun. 1986, 1325–1327;
- 2fC. O. Dietrich-Buchecker, A. Khemiss, J.-P. Sauvage, J. Chem. Soc. Chem. Commun. 1986, 1376–1378;
- 2gC. O. Dietrich-Buchecker, J.-P. Sauvage, Tetrahedron 1990, 46, 503–512;
- 2hB. Mohr, J.-P. Sauvage, R. H. Grubbs, M. Weck, Angew. Chem. Int. Ed. Engl. 1997, 36, 1308–1310; Angew. Chem. 1997, 109, 1365–1367;
- 2iP. Gaviña, J.-P. Sauvage, Tetrahedron Lett. 1997, 38, 3521–3524;
- 2jM. Weck, B. Mohr, J.-P. Sauvage, R. H. Grubbs, J. Org. Chem. 1999, 64, 5463–5471;
- 2kP. Mobian, J.-M. Kern, J.-P. Sauvage, J. Am. Chem. Soc. 2003, 125, 2016–2017.
- 3
- 3aC. O. Dietrich-Buchecker, J.-P. Sauvage, Angew. Chem. Int. Ed. Engl. 1989, 28, 189–192; Angew. Chem. 1989, 101, 192–194;
- 3bC. O. Dietrich-Buchecker, J. Guilhem, C. Pascard, J.-P. Sauvage, Angew. Chem. Int. Ed. Engl. 1990, 29, 1154–1156; Angew. Chem. 1990, 102, 1202–1204;
- 3cC. O. Dietrich-Buchecker, J.-F. Nierengarten, J.-P. Sauvage, N. Armaroli, V. Balzani, L. De Cola, J. Am. Chem. Soc. 1993, 115, 11237–11244;
- 3dC. O. Dietrich-Buchecker, G. Rapenne, J.-P. Sauvage, J. Chem. Soc. Chem. Commun. 1997, 2053–2054;
- 3eP. E. Barran, H. L. Cole, S. M. Goldup, D. A. Leigh, P. R. McGonigal, M. D. Symes, J. Wu, M. Zengerle, Angew. Chem. Int. Ed. 2011, 50, 12280–12284; Angew. Chem. 2011, 123, 12488–12492;
- 3fJ.-F. Ayme, J. E. Beves, D. A. Leigh, R. T. McBurney, K. Rissanen, D. Schultz, Nat. Chem. 2012, 4, 15–20;
- 3gJ.-F. Ayme, J. E. Beves, C. J. Campbella, D. A. Leigh, Chem. Soc. Rev. 2013, 42, 1700–1712;
- 3hD. A. Leigh, R. G. Pritchard, A. J. Stephens, Nat. Chem. 2014, 6, 978–982.
- 4
- 4aA. Livoreil, C. O. Dietrich-Buchecker, J.-P. Sauvage, J. Am. Chem. Soc. 1994, 116, 9399–9400;
- 4bN. Armaroli, V. Balzani, J.-P. Collin, P. Gaviña, J.-P. Sauvage, B. Ventura, J. Am. Chem. Soc. 1999, 121, 4397–4408;
- 4cM.-J. Blanco, M. C. Jiménez, J.-C. Chambron, V. Heitz, M. Linke, J.-P. Sauvage, Chem. Soc. Rev. 1999, 28, 293–305;
- 4dM. C. Jiménez, C. O. Dietrich-Buchecker, J.-P. Sauvage, Angew. Chem. Int. Ed. 2000, 39, 3284–3287;
10.1002/1521-3773(20000915)39:18<3284::AID-ANIE3284>3.0.CO;2-7 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 3422–3425;
- 4eI. Poleschak, J.-M. Kern, J.-P. Sauvage, Chem. Commun. 2004, 474–476;
- 4fP. Mobian, J.-M. Kern, J.-P. Sauvage, Angew. Chem. Int. Ed. 2004, 43, 2392–2395; Angew. Chem. 2004, 116, 2446–2449;
- 4gS. Bonnet, J.-P. Collin, M. Koizumi, P. Mobian, J.-P. Sauvage, Adv. Mater. 2006, 18, 1239–1250.
- 5
- 5aM. J. Langton, P. D. Beer, Acc. Chem. Res. 2014, 47, 1935–1949;
- 5bK. Hiratani, M. Kaneyama, Y. Nagawa, E. Koyama, M. Kanesato, J. Am. Chem. Soc. 2004, 126, 13568–13569;
- 5cY. Nagawa, J. Suga, K. Hiratani, E. Koyama, M. Kanesato, Chem. Commun. 2005, 749–751;
- 5dY. Nakatani, Y. Furusho, E. Yashima, Angew. Chem. Int. Ed. 2010, 49, 5463–5467; Angew. Chem. 2010, 122, 5595–5599;
- 5eS.-Y. Hsueh, C.-C. Lai, S.-H. Chiu, Chem. Eur. J. 2010, 16, 2997–3000;
- 5fA. V. Leontiev, C. J. Serpell, N. G. White, P. D. Beer, Chem. Sci. 2011, 2, 922–927;
- 5gN.-C. Chen, P.-Y. Huang, C.-C. Lai, Y.-H. Liu, Y. Wang, S.-M. Peng, S.-H. Chiu, Chem. Commun. 2007, 4122–4124;
- 5hN.-C. Chen, C.-C. Lai, Y.-H. Liu, S.-M. Peng, S.-H. Chiu, Chem. Eur. J. 2008, 14, 2904–2908.
- 6
- 6aE. A. Neal, S. M. Goldup, Chem. Commun. 2014, 50, 5128–5142;
- 6bD. A. Leigh, V. Marcos, M. R. Wilson, ACS Catal. 2014, 4, 4490–4497;
- 6cY. Tachibana, N. Kihara, T. Takata, J. Am. Chem. Soc. 2004, 126, 3438–3439;
- 6dY. Suzaki, K. Shimada, E. Chihara, T. Saito, Y. Tsuchido, K. Osakada, Org. Lett. 2011, 13, 3774–3777;
- 6eC. B. Caputo, K. Zhu, V. N. Vukotic, S. J. Loeb, D. W. Stephan, Angew. Chem. Int. Ed. 2013, 52, 960–963; Angew. Chem. 2013, 125, 994–997;
- 6fB. Lewandowski, G. De Bo, J. W. Ward, M. Papmeyer, S. Kuschel, M. J. Aldegunde, P. M. E. Gramlich, D. Heckmann, S. M. Goldup, D. M. D′Souza, A. E. Fernandes, D. A. Leigh, Science 2013, 339, 189–193;
- 6gM. Galli, J. E. M. Lewis, S. M. Goldup, Angew. Chem. Int. Ed. 2015, 54, 13545–13549; Angew. Chem. 2015, 127, 13749–13753.
- 7
- 7aK. Zhu, V. N. Vukotic, C. A. O'Keefe, R. W. Schurko, S. J. Loeb, J. Am. Chem. Soc. 2014, 136, 7403–7409;
- 7bK. Zhu, V. N. Vukotic, C. A. O'Keefe, R. W. Schurko, S. J. Loeb, Nat. Chem. 2015, 7, 514–519;
- 7cV. N. Vukotic, C. A. O'Keefe, K. Zhu, K. J. Harris, C. To, R. W. Schurko, S. J. Loeb, J. Am. Chem. Soc. 2015, 137, 9643–9651;
- 7dN. Farahani, K. Zhu, C. A. O'Keefe, R. W. Schurko, S. J. Loeb, ChemPlusChem 2016, 81, 836–841.
- 8
- 8aK. Zhu, V. N. Vukotic, S. J. Loeb, Angew. Chem. Int. Ed. 2012, 51, 2168–2172; Angew. Chem. 2012, 124, 2210–2214;
- 8bN. Noujeim, K. Zhu, V. N. Vukotic, S. J. Loeb, Org. Lett. 2012, 14, 2484–2487;
- 8cK. Zhu, V. N. Vukotic, N. Noujeim, S. J. Loeb, Chem. Sci. 2012, 3, 3265–3271;
- 8dN. Farahani, K. Zhu, S. J. Loeb, ChemPhysChem 2016, 17, 1875–1880.
- 9
- 9aA. F. M. Kilbinger, S. J. Cantrill, A. W. Waltman, M. W. Day, R. H. Grubbs, Angew. Chem. Int. Ed. 2003, 42, 3281–3285; Angew. Chem. 2003, 115, 3403–3407;
- 9bV. N. Vukotic, K. J. Harris, K. Zhu, R. W. Schurko, S. J. Loeb, Nat. Chem. 2012, 4, 456–460.
- 10
- 10aM. Berthelot, Ann. Chim. Phys. 1901, 23, 32–39;
- 10bW. Manchot, W. Brandt, Justus Liebigs Ann. Chem. 1909, 370, 286–296;
10.1002/jlac.19093700303 Google Scholar
- 10cH. Tropsch, W. J. Mattox, J. Am. Chem. Soc. 1935, 57, 1102–1103;
- 10dE. R. Gilliland, H. L. Bliss, C. E. Kip, J. Am. Chem. Soc. 1941, 63, 2088–2090;
- 10eM. J. S. Dewer, Bull. Soc. Chim. Fr. 1951, 18, C 79;
- 10fM. A. Bennett, Chem. Rev. 1962, 62, 611–652;
- 10gR. G. Salomon, J. K. Kochi, J. Am. Chem. Soc. 1973, 95, 1889–1897;
- 10hJ. S. Thompson, R. L. Harlow, J. F. Whitney, J. Am. Chem. Soc. 1983, 105, 3522–3527;
- 10iM. Munakata, S. Kitagawa, S. Kosome, A. Asahara, Inorg. Chem. 1986, 25, 2622–2627;
- 10jX.-S. Wang, H. Zhao, Y.-H. Li, R.-G. Xiong, X.-Z. You, Top. Catal. 2005, 35, 43–61.
- 11
- 11aD. J. Safarik, R. B. Eldridge, Ind. Eng. Chem. Res. 1998, 37, 2571–2581;
- 11bA. Takahashi, R. T. Yang, Langmuir 2001, 17, 8405–8413;
- 11cE. I. Basaldella, J. C. Tara, G. Aguilar Armenta, M. E. Patiño-Iglesias, E. Rodríguez Castellon, J. Sol-Gel Sci. Technol. 2006, 37, 141–146.
- 12
- 12aJ. M. Cubero, H. K. Mangold, Microchem. J. 1965, 9, 227–236;
- 12bB. M. Lawrence, J. Chromatogr. A 1968, 38, 535–537;
- 12cT.-S. Li, J.-T. Li, H.-Z. Li, J. Chromatogr. A 1995, 715, 372–375;
- 12dC. M. Williams, L. N. Mander, Tetrahedron 2001, 57, 425–447.
- 13Along with cis and trans isomers of the double bond, there are possible cisoid and transoid conformations of the N,N′-imidazole chelate. The latter are easily interconverted by rotation about the single bond between the imidazole groups. Only the cisoid, N,N′-imidazole chelate is used to bind to a metal cation.
- 14CCDC 1496411–1496414 contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.