Metal-Free Activation of Hydrogen, Carbon Dioxide, and Ammonia by the Open-Shell Singlet Biradicaloid [P(μ-NTer)]2
Dr. Alexander Hinz
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, OX1 3TA Oxford, UK
Search for more papers by this authorCorresponding Author
Prof. Dr. Axel Schulz
Institut für Chemie, Universität Rostock, Albert-Einstein-Strasse 3a, 18059 Rostock, Germany
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Alexander Villinger
Institut für Chemie, Universität Rostock, Albert-Einstein-Strasse 3a, 18059 Rostock, Germany
Search for more papers by this authorDr. Alexander Hinz
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, 12 Mansfield Road, OX1 3TA Oxford, UK
Search for more papers by this authorCorresponding Author
Prof. Dr. Axel Schulz
Institut für Chemie, Universität Rostock, Albert-Einstein-Strasse 3a, 18059 Rostock, Germany
Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
Search for more papers by this authorDr. Alexander Villinger
Institut für Chemie, Universität Rostock, Albert-Einstein-Strasse 3a, 18059 Rostock, Germany
Search for more papers by this authorGraphical Abstract
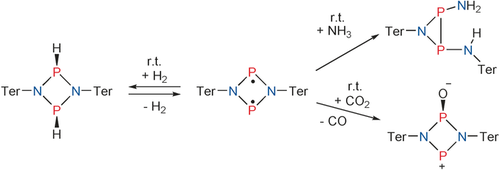
Don't wait for activation: The singlet biradicaloid [P(μ-NTer)]2 readily reacts with H2, CO2, or NH3 at ambient temperature. The addition of H2 is reversible whereas CO2 is reduced to CO with formation of “biradicaloid monoxide”. Activation of ammonia causes the P2N2 scaffold to rearrange to give an azadiphosphiridine.
Abstract
The Group 15 open-shell singlet biradicaloid [P(μ-NTer)]2 (Ter=2,6-bis(2,4,6-trimethylphenyl)phenyl) was utilized in the activation of stable small molecules. Fast reactions with H2, CO2, and NH3 were observed. Dihydrogen easily added to [P(μ-NTer)]2 , yielding [HP(μ-NTer)]2 under ambient conditions whereas reversible release of molecular hydrogen was observed at slightly elevated temperatures (T>60 °C). As [P(μ-NTer)]2 is a species with phosphorus in the unusual formal oxidation state +II, it is capable of reducing carbon dioxide to afford a zwitterionic compound, [OP(μ-NTer)2P], and carbon monoxide. The reaction of [P(μ-NTer)]2 with ammonia led to the formation of an azadiphosphiridine after rearrangements of the central P2N2 heterocycle.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201606892-sup-0001-misc_information.pdf741.1 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2010, 49, 46–76; Angew. Chem. 2010, 122, 50–81.
- 2T. Sakakura, J. C. Choi, H. Yasuda, Chem. Rev. 2007, 107, 2365–2387.
- 3J. I. van der Vlugt, Chem. Soc. Rev. 2010, 39, 2302–2322.
- 4G. Linti, H. Schnöckel, Coord. Chem. Rev. 2000, 206–207, 285–319.
- 5D. Martin, M. Soleilhavoup, G. Bertrand, Chem. Sci. 2011, 2, 389–399.
- 6M. Soleilhavoup, G. Bertrand, Acc. Chem. Res. 2015, 48, 256–266.
- 7P. P. Power, Chem. Rev. 2003, 103, 789–809.
- 8G. He, O. Shynkaruk, M. W. Lui, E. Rivard, Chem. Rev. 2014, 114, 7815–7880.
- 9M. Abe, Chem. Rev. 2013, 113, 7011–7088.
- 10R. Fischer, P. P. Power, Chem. Rev. 2010, 110, 3877–3923.
- 11P. P. Power, Nature 2010, 463, 171–177.
- 12G. C. Welch, R. R. S. Juan, J. D. Masuda, D. W. Stephan, Science 2006, 314, 1124–1126.
- 13C. Appelt, J. C. Slootweg, K. Lammertsma, W. Uhl, Angew. Chem. Int. Ed. 2013, 52, 4256–4259; Angew. Chem. 2013, 125, 4350–4353.
- 14F. Hengesbach, X. Jin, A. Hepp, B. Wibbeling, E. U. Würthwein, W. Uhl, Chem. Eur. J. 2013, 19, 13901–13909.
- 15D. W. Stephan, G. Erker, Chem. Sci. 2014, 5, 2625.
- 16G. D. Frey, V. Lavallo, B. Donnadieu, W. W. Schoeller, G. Bertrand, Science 2007, 316, 439–441.
- 17U. Siemeling, C. Färber, C. Bruhn, M. Leibold, D. Selent, W. Baumann, M. von Hopffgarten, C. Goedecke, G. Frenking, Chem. Sci. 2010, 1, 697–704.
- 18A. Schäfer, M. Reißmann, A. Schäfer, M. Schmidtmann, T. Müller, Chem. Eur. J. 2014, 20, 9381–9386.
- 19A. Jana, C. Schulzke, H. W. Roesky, J. Am. Chem. Soc. 2009, 131, 4600–4601.
- 20Z. D. Brown, P. P. Power, Inorg. Chem. 2013, 52, 6248–6259.
- 21P. Vasko, S. Wang, H. M. Tuononen, P. P. Power, Angew. Chem. Int. Ed. 2015, 54, 3802–3805; Angew. Chem. 2015, 127, 3873–3876.
- 22M. Stoelzel, S. Inoue, A. Meltzer, M. Driess, Angew. Chem. Int. Ed. 2010, 49, 10002–10005; Angew. Chem. 2010, 122, 10199–10202.
- 23K. Hansen, T. Szilvási, B. Blom, E. Irran, M. Driess, Chem. Eur. J. 2014, 20, 1947–1956.
- 24J. A. B. Abdalla, I. M. Riddlestone, R. Tirfoin, S. Aldridge, Angew. Chem. Int. Ed. 2015, 54, 5098–5102; Angew. Chem. 2015, 127, 5187–5191.
- 25W. Zhao, S. M. McCarthy, T. Y. Lai, H. P. Yennawar, A. T. Radosevich, J. Am. Chem. Soc. 2014, 136, 17634–17644.
- 26N. L. Dunn, M. Ha, A. T. Radosevich, J. Am. Chem. Soc. 2012, 134, 11330–11333.
- 27T. P. Robinson, D. M. De Rosa, S. Aldridge, J. M. Goicoechea, Angew. Chem. Int. Ed. 2015, 54, 13758–13763; Angew. Chem. 2015, 127, 13962–13967.
- 28Z. Zhu, X. Wang, Y. Peng, H. Lei, J. C. Fettinger, E. Rivard, P. P. Power, Angew. Chem. Int. Ed. 2009, 48, 2031–2034; Angew. Chem. 2009, 121, 2065–2068.
- 29A. Seifert, D. Scheid, G. Linti, T. Zessin, Chem. Eur. J. 2009, 15, 12114–12120.
- 30M. Hermann, C. Jones, G. Frenking, Inorg. Chem. 2014, 53, 6482–6490.
- 31G. H. Spikes, J. C. Fettinger, P. P. Power, J. Am. Chem. Soc. 2005, 127, 12232–12233.
- 32G. H. Spikes, Y. Peng, J. C. Fettinger, J. Steiner, P. P. Power, Chem. Commun. 2005, 6041–6043.
- 33X. Wang, Y. Peng, M. M. Olmstead, J. C. Fettinger, P. P. Power, J. Am. Chem. Soc. 2009, 131, 14164–14165.
- 34Y. Jung, M. Brynda, P. P. Power, M. Head-Gordon, J. Am. Chem. Soc. 2006, 128, 7185–7192.
- 35L. Zhao, F. Huang, G. Lu, Z. X. Wang, P. V. R. Schleyer, J. Am. Chem. Soc. 2012, 134, 8856–8868.
- 36J. Moilanen, P. P. Power, H. M. Tuononen, Inorg. Chem. 2010, 49, 10992–11000.
- 37C. A. Caputo, P. P. Power, Organometallics 2013, 32, 2278–2286.
- 38C. A. Caputo, J. Koivistoinen, J. Moilanen, J. N. Boynton, H. M. Tuononen, P. P. Power, J. Am. Chem. Soc. 2013, 135, 1952–1960.
- 39In this work, the term “biradicaloid” refers to a biradical in which the radical centers interact significantly and adopt a singlet ground state. For further information on biradical(oid)s, see:
- 39aRef. [9];
- 39bF. Breher, Coord. Chem. Rev. 2007, 251, 1007–1043.
- 40T. Beweries, R. Kuzora, U. Rosenthal, A. Schulz, A. Villinger, Angew. Chem. Int. Ed. 2011, 50, 8974–8978; Angew. Chem. 2011, 123, 9136–9140.
- 41S. Demeshko, C. Godemann, R. Kuzora, A. Schulz, A. Villinger, Angew. Chem. Int. Ed. 2013, 52, 2105–2108; Angew. Chem. 2013, 125, 2159–2162.
- 42A. Hinz, A. Schulz, A. Villinger, Angew. Chem. Int. Ed. 2015, 54, 668–672; Angew. Chem. 2015, 127, 678–682.
- 43A. Hinz, R. Kuzora, U. Rosenthal, A. Schulz, A. Villinger, Chem. Eur. J. 2014, 20, 14659–14673.
- 44A. Hinz, A. Schulz, W. W. Seidel, A. Villinger, Inorg. Chem. 2014, 53, 11682–11690.
- 45A. Hinz, A. Schulz, A. Villinger, J.-M. Wolter, J. Am. Chem. Soc. 2015, 137, 3975–3980.
- 46R. Batchelor, T. Birchall, J. Am. Chem. Soc. 1982, 104, 674–679.
- 47Y. Tantirungrotechai, K. Phanasant, S. Roddecha, P. Surawatanawong, V. Sutthikhum, J. Limtrakul, J. Mol. Struct. THEOCHEM 2006, 760, 189–192.
- 48A. Hinz, A. Schulz, Phosphorus Sulfur Relat. Elem. 2016, 191, 578–581.
- 49Compound 4 can also be prepared by reaction of 1 with TEMPO. Details are given in the Supporting Information.
- 50V. Schomaker, D. P. Stevenson, J. Am. Chem. Soc. 1941, 63, 37–40.
- 51P. Pyykkö, M. Atsumi, Chem. Eur. J. 2009, 15, 12770–12779.
- 52M. J. Frisch et al., Gaussian 09 D.01, Gaussian, Inc., Wallingford CT, 2009.
- 53A. H. Cowley, M. Lattman, J. C. Wilburn, Inorg. Chem. 1981, 20, 2916–2919.
- 54D. Michalik, A. Schulz, A. Villinger, N. Weding, Angew. Chem. Int. Ed. 2008, 47, 6465–6468; Angew. Chem. 2008, 120, 6565–6568.
- 55A second possible reaction pathway involves the attack of both O atoms of the CO2 molecule at one P atom leading to the formation of a four-membered spirocyclic intermediate (spiro-3Ph; Table S5 and Figure S8), which, however, is not favored as the energy barrier for the first transition state is much higher (3Ph: 22.7 vs. spiro-3Ph: 60.3 kcal mol−1), and spiro-3Ph represents a high-lying isomer (ΔG°298=49.8 kcal mol−1).
- 56E. Niecke, A. Nickloweit-Lüke, R. Rüger, Angew. Chem. Int. Ed. Engl. 1981, 20, 385–386; Angew. Chem. 1981, 93, 406–407.
- 57E. Niecke, A. Nickloweit-Lüke, R. Rüger, B. Krebs, H. Grewe, Z. Naturforsch. B 1981, 36, 1566–1574.
- 58R. Streubel, E. Niecke, P. Paetzold, Chem. Ber. 1991, 124, 765–767.
- 59D. Barion, C. Gärtner-Winkhaus, M. Link, M. Nieger, E. Niecke, Chem. Ber. 1993, 126, 2187–2195.
- 60E. Niecke, B. Kramer, M. Nieger, H. Severin, Tetrahedron Lett. 1993, 34, 4627–4630.
- 61A. Hinz, A. Schulz, A. Villinger, Chem. Commun. 2015, 51, 1363–1366.