In Situ Observation of Hydrogen-Induced Surface Faceting for Palladium–Copper Nanocrystals at Atmospheric Pressure
Ying Jiang
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorHengbo Li
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorZhemin Wu
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorWenying Ye
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorProf. Hui Zhang
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Yong Wang
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Dr. Chenghua Sun
ARC Centre for Electromaterials Science, School of Chemistry, Monash University, Clayton, Victoria, 3800 Australia
Search for more papers by this authorProf. Ze Zhang
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorYing Jiang
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorHengbo Li
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
These authors contributed equally to this work.
Search for more papers by this authorZhemin Wu
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorWenying Ye
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorProf. Hui Zhang
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Prof. Yong Wang
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorCorresponding Author
Dr. Chenghua Sun
ARC Centre for Electromaterials Science, School of Chemistry, Monash University, Clayton, Victoria, 3800 Australia
Search for more papers by this authorProf. Ze Zhang
State Key Laboratory of Silicon Materials and Center of Electron Microscopy, School of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027 China
Search for more papers by this authorGraphical Abstract
Abstract
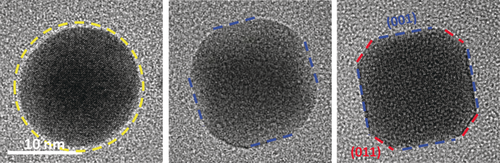
Nanocrystal (NC) morphology, which decides the number of active sites and catalytic efficiency, is strongly determined by the gases involved in synthesis, treatment, and reaction. Myriad investigations have been performed to understand the morphological response to the involved gases. However, most prior work is limited to low pressures, which is far beyond realistic conditions. A dynamic morphological evolution of palladium–copper (PdCu) NC within a nanoreactor is reported, with atmospheric pressure hydrogen at the atomic scale. In situ transmission electron microscopy (TEM) videos reveal that spherical PdCu particles transform into truncated cubes at high hydrogen pressure. First principles calculations demonstrate that the surface energies decline with hydrogen pressure, with a new order of γH-001<γH-110<γH-111 at 1 bar. A comprehensive Wulff construction based on the corrected surface energies is perfectly consistent with the experiments. The work provides a microscopic insight into NC behaviors at realistic gas pressure and is promising for the shaping of nanocatalysts by gas-assisted treatments.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201605956-sup-0001-misc_information.pdf1.2 MB | Supplementary |
| anie201605956-sup-0001-MovieS1.avi7.2 MB | Supplementary |
| anie201605956-sup-0001-MovieS2.avi9.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aQ. Gao, Y.-M. Ju, D. An, M.-R. Gao, C.-H. Cui, J.-W. Liu, H.-P. Cong, S.-H. Yu, ChemSusChem 2013, 6, 1878;
- 1bH. Lee, S. E. Habas, S. Kweskin, D. Butcher, G. A. Somorjai, P. Yang, Angew. Chem. Int. Ed. 2006, 45, 7824; Angew. Chem. 2006, 118, 7988;
- 1cL. Wang, S. Zhao, C. Liu, C. Li, X. Li, H. Li, Y. Wang, C. Ma, Z. Li, J. Zeng, Nano Lett. 2015, 15, 2875.
- 2
- 2aY. Dai, X. Mu, Y. Tan, K. Lin, Z. Yang, N. Zheng, G. Fu, J. Am. Chem. Soc. 2012, 134, 7073;
- 2bT. S. Ahmadi, Z. L. Wang, T. C. Green, A. Henglein, M. A. El-Sayed, Science 1996, 272, 1924;
- 2cJ. Ren, R. D. Tilley, Small 2007, 3, 1508.
- 3
- 3aF. Gao, Y. Wang, D. W. Goodman, J. Phys. Chem. C 2009, 113, 14993;
- 3bL.-L. Wang, T. L. Tan, D. D. Johnson, Nano Lett. 2014, 14, 7077;
- 3cM. Chi, C. Wang, Y. Lei, G. Wang, D. Li, K. L. More, A. Lupini, L. F. Allard, N. M. Markovic, V. R. Stamenkovic, Nat. Commun. 2015, 6, 8925;
- 3dS. B. Vendelbo, C. F. Elkjær, H. Falsig, I. Puspitasari, P. Dona, L. Mele, B. Morana, B. J. Nelissen, R. van Rijn, J. F. Creemer, P. J. Kooyman, S. Helveg, Nat. Mater. 2014, 13, 884;
- 3eW. Yuan, Y. Wang, H. Li, H. Wu, Z. Zhang, A. Selloni, C. Sun, Nano Lett. 2016, 16, 132;
- 3fY. A. Wu, L. Li, Z. Li, A. Kinaci, M. K. Y. Chan, Y. Sun, J. R. Guest, I. McNulty, T. Rajh, Y. Liu, ACS Nano 2016, 10, 3738;
- 3gH. Li, W. Yuan, Y. Jiang, Z. Zhang, Z. Zhang, Y. Wang, Prog. Nat. Sci.: Mater. Int. 2016, 26, 308;
- 3hE. de Smit, I. Swart, J. F. Creemer, C. Karunakaran, D. Bertwistle, H. W. Zandbergen, F. M. F. de Groot, B. M. Weckhuysen, Angew. Chem. Int. Ed. 2009, 48, 3632; Angew. Chem. 2009, 121, 3686;
- 3iH. Yoshida, Y. Kuwauchi, J. R. Jinschek, K. J. Sun, S. Tanaka, M. Kohyama, S. Shimada, M. Haruta, S. Takeda, Science 2012, 335, 317;
- 3jS. Y. Zhang, P. N. Plessow, J. J. Willis, S. Dai, M. J. Xu, G. W. Graham, M. Cargnello, F. Abild-Pedersen, X. Q. Pan, Nano Lett. 2016, 16, 4528;
- 3kA. Baldi, T. C. Narayan, A. L. Koh, J. A. Dionne, Nat. Mater. 2014, 13, 1143.
- 4P. Nolte, A. Stierle, N. Y. Jin-Phillipp, N. Kasper, T. U. Schulli, H. Dosch, Science 2008, 321, 1654.
- 5P. L. Hansen, J. B. Wagner, S. Helveg, J. R. Rostrup-Nielsen, B. S. Clausen, H. Topsøe, Science 2002, 295, 2053.
- 6H. L. Xin, S. Alayoglu, R. Tao, A. Genc, C.-M. Wang, L. Kovarik, E. A. Stach, L.-W. Wang, M. Salmeron, G. A. Somorjai, H. Zheng, Nano Lett. 2014, 14, 3203.
- 7F. Tao, S. Dag, L.-W. Wang, Z. Liu, D. R. Butcher, H. Bluhm, M. Salmeron, G. A. Somorjai, Science 2010, 327, 850.
- 8
- 8aB. D. Adams, A. Chen, Mater. Today 2011, 14, 282;
- 8bR. Dittmeyer, V. Hollein, K. Daub, J. Mol. Catal. A 2001, 173, 135;
- 8cY. Bi, H. Xu, W. Li, A. Goldbach, Int. J. Hydrogen Energy 2009, 34, 2965;
- 8dJ. Shu, B. P. A. Grandjean, S. Kaliaguine, Appl. Catal. A 1994, 119, 305;
- 8eX. Jiang, N. Koizumi, X. Guo, C. Song, Appl. Catal. B 2015, 170, 173.
- 9
- 9aJ. Mao, Y. Liu, Z. Chen, D. Wang, Y. Li, Chem. Commun. 2014, 50, 4588;
- 9bK.-H. Park, Y. W. Lee, S. W. Kang, S. W. Han, Chem. Asian J. 2011, 6, 1515;
- 9cE. B. Fox, S. Velu, M. H. Engelhard, Y.-H. Chin, J. T. Miller, J. Kropf, C. Song, J. Catal. 2008, 260, 358;
- 9dB. Jiang, C. Li, V. Malgras, Y. Bando, Y. Yamauchi, Chem. Commun. 2016, 52, 1186;
- 9eM.-W. Hsieh, T.-J. Whang, Appl. Surf. Sci. 2013, 270, 252.
- 10J. F. Creemer, S. Helveg, G. H. Hoveling, S. Ullmann, A. M. Molenbroek, P. M. Sarro, H. W. Zandbergen, Ultramicroscopy 2008, 108, 993.
- 11
- 11aM. Yamauchi, T. Tsukuda, Dalton Trans. 2011, 40, 4842;
- 11bC. Wang, D. P. Chen, X. Sang, R. R. Unocic, S. E. Skrabalak, ACS Nano 2016, 10, 6345.
- 12Y. Jiang, Y. Wang, Y. Zhang, Z. Zhang, W. Yuan, C. Sun, X. Wei, C. Brodsky, C.-K. Tsung, J. Li, X. Zhang, S. Mao, S. Zhang, Z. Zhang, Nano Res. 2014, 7, 308.
- 13D. G. Narehood, S. Kishore, H. Goto, J. H. Adair, Int. J. Hydrogen Energy 2009, 34, 952.
- 14S. M. Opalka, W. Huang, D. Wang, T. B. Flanagan, O. M. Lovvik, S. C. Emerson, Y. She, T. H. Vanderspurt, J. Alloys Compd. 2007, 446, 583.
- 15B. Zhu, Z. Xu, C. Wang, Y. Gao, Nano Lett. 2016, 16, 2628.
- 16
- 16aG. L. Kellogg, Phys. Rev. B 1997, 55, 7206;
- 16bS. Horch, H. T. Lorensen, S. Helveg, E. Laegsgaard, I. Stensgaard, K. W. Jacobsen, J. K. Norskov, F. Besenbacher, Nature 1999, 398, 134.