Polynuclear Gold [AuI]4, [AuI]8, and Bimetallic [AuI4AgI] Complexes: C−H Functionalization of Carbonyl Compounds and Homogeneous Carbonylation of Amines
Graphical Abstract
Gold aplenty: The synthesis of tetranuclear gold complexes, a structurally unprecedented octanuclear complex with a planar [AuI8] core, and pentanuclear [AuI4MI] (M=Cu, Ag) complexes is presented. The linear [AuI4] complex undergoes C−H functionalization of carbonyl compounds under mild reaction conditions. In addition, [AuI4AgI] catalyzes the carbonylation of primary amines to form ureas under homogeneous conditions with efficiencies higher than those achieved by gold nanoparticles.
Abstract
The synthesis of tetranuclear gold complexes, a structurally unprecedented octanuclear complex with a planar [AuI8] core, and pentanuclear [AuI4MI] (M=Cu, Ag) complexes is presented. The linear [AuI4] complex undergoes C−H functionalization of carbonyl compounds under mild reaction conditions. In addition, [AuI4AgI] catalyzes the carbonylation of primary amines to form ureas under homogeneous conditions with efficiencies higher than those achieved by gold nanoparticles.
Following the pioneering work of the groups of Hutchings1 and Haruta2 on the oxidation of hydrocarbons and CO, many gold clusters and nanoparticles have been structurally characterized and studied in catalysis.3, 4 Recently, small gold clusters have been found to activate the C−I bond of iodobenzene in the gas phase5 and to catalyze reactions usually performed by mononuclear gold complexes.6 However, the actual structures of these clusters have not been determined. In this context, it is important to note that the group of Bertrand reported the synthesis of mixed-valence trinuclear [Au02AuI] clusters which are catalysts for the carbonylation of aliphatic amines under homogeneous conditions.7
As part of our program on the study of the catalytic activity of well-defined gold(I) clusters,8 we now report a ready synthesis of linear [Au4], pentagonal [AuI4MI] (M=Cu, Ag), and octagonal [Au8] complexes. The linear [Au4] complex reacts with carbonyl compounds by C−H functionalization to form square-planar complexes. Furthermore, the pentanuclear complex [AuI4AgI] has been found to be an excellent homogeneous catalyst for the carbonylation of amines.
Our synthesis started from the dinuclear gold(I) complex 1, which is quantitatively prepared by mixing 2,6-bis(diphenylphosphino)pyridine [(Ph2P)2Py]9 with 2 equivalents of [AuCl(tht)] (Scheme 1). Upon heating to 130 °C in 1,1,2,2-tetrachloroethane, the initial insoluble white solid gives a colorless solution,10, 11 which was treated with 1 or 2 equivalents of AgSbF6 in MeCN at 25 °C to give tetranuclear complexes 2 and 3, respectively, in good yields.12, 13 The structures of both complexes were determined by X-ray diffraction,14 which shows the two tridentate ligands trans to each other and aligned with one-unit translation.15 In the solid state, 2 forms a dimeric [Au4]2 structure stabilized by aurophilic interactions (3.24 Å distance between two terminal gold atoms in each of Au4 fragments) along with rare intermolecular Au⋅⋅⋅Cl interactions (3.38 Å).16 However, in solution, two singlets were observed at δ=42.3 and 38.0 ppm in the 31P{1H} NMR spectrum of monomeric [Au4], which has two identical P-Au-Cl and P-Au-N units.
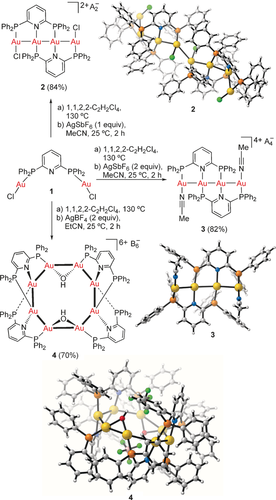
Synthesis of the [Au4] complexes 2 and 3 and the [Au8] complex 4. A=SbF6, B=BF4.
Remarkably, when EtCN was used instead of MeCN, and the ratio of nitrile to 1,1,2,2-tetrachloroethane was reduced from 1:3.5 to 1:25, the new complex 4 was obtained and showed a mass ion at m/z 1219.3998 (calcd 1219.4228) in MALDI-TOF, which corresponds to the octanuclear {[Au8(OH)2L4](BF4)3}3+ [L=((Ph2P)2Py)]17, 18. X-Ray diffraction of 414 revealed an unprecedented planar octagonal [Au8] structure,19 which is very different from those reported for mixed-valence {Au0AuI} octanuclear clusters.20 The complex 4 consists of eight AuI centers bound to the next one by the bridging ligands and aurophilic interactions. All Au⋅⋅⋅Au contacts are within 2.81–3.00 Å, thus corresponding to bond orders on the order of 0.2 according to DFT calculations,21 which are similar to those determined experimentally by X-ray diffraction (2.80–3.08 Å).
When complex 3 was dissolved in a mixture of acetone and pentane, the complex 5 a, having two Au–CH2COMe units, was slowly formed, although for preparative purposes the reaction performed better in the presence of Ag2O as a base (Scheme 2). In this process a remarkable reorganization from a linear to square-planar C2-chiral structure has taken place.22 The Au–CH2 bond distance of 2.09 Å, determined by X-ray diffraction,14 is similar to that found in other gold(I) C-enolates of acetone.23 The complex 3 also reacted with 1,3-indandione, 1,3-cyclohexadienone, and Meldrum's acid to form the complexes 5 b–d in good yields by C−H functionalization, which showed Au−C bonds in the 2.05–2.07 Å range.14 Related C−H functionalizations of methyl ketones have been observed with other gold complexes,24 as well as with an oxo AuI–AgI cluster.25
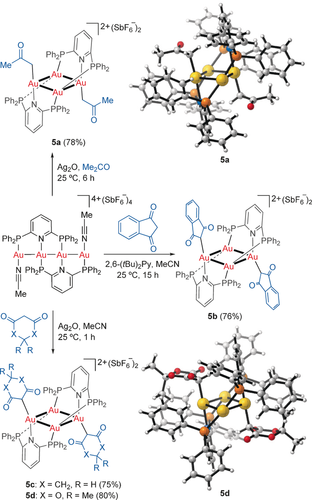
C−H functionalization of carbonyl compounds with 3.
To gain insight into the mechanism of this C−H functionalization, we performed the reaction of 3 with Cu2O or Ag2O in MeCN in the absence of carbonyl compounds. Interestingly, the new C2-chiral complexes 6 a and 7 a, with a trapezoid pentanuclear [Au4M] core, were cleanly obtained in 68 and 75 % yield, respectively (Scheme 3).14 Remarkably, the acetonitrile ligands of 3 had suffered hydrolysis by trace amounts of water, in the solvent, under unusually mild reaction conditions to form the bridged acetate ligands of 6 a and 7 a. Similarly, reaction of 3 with either Cu2O or Ag2O in EtCN (HPLC grade) led to the corresponding 6 b and 7 b with propionate bridges in 75 and 80 %, respectively. The complexes 6 a, 7 a, and 7 c were also readily obtained by reaction of 3 with copper(I) and silver(I) carboxylates in a 4+1-type process. The complexes 6 a and 7 a display significant aurophilic as well as Au–Cu or Au–Ag interactions and are the first examples in which a carboxylate ligand bridges between AuI and either AgI or CuI.
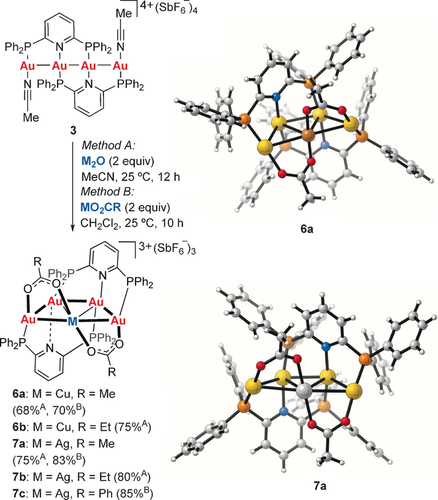
Synthesis of the [Au4M] complexes 6 and 7.
We also tested the new complexes as catalysts for the carbonylative synthesis of ureas from primary amines under the reaction conditions reported by Bertrand with a mixed-valence trinuclear [Au02AuI] cluster.7 This report is significant because all other gold-catalyzed carbonylations of amines have been carried out under heterogeneous conditions.26 Thus, although the first gold-catalyzed synthesis of urea by carbonylation of aliphatic amines was performed with [AuCl(PPh3)],27 under the harsh reaction conditions (200 °C) gold nanoparticles are actually formed.28 Bulk gold also gives ureas via intermediate isocyanates,29 whereas gold nanoparticles promote the cyclocarbonylation of 2-aminophenols to 2-benzoxazolinones.30 For our study, we chose cyclohexylamine as the substrate with the catalyst (2 mol %) dissolved in THF in a vial open to the air, and then 5 bar of CO was introduced and maintained in a low-pressure reactor, at 60 °C for 24 hours. The complexes 3, 6 a,b, 7 b,c gave the urea 8 a in low yields (Table 1, entries 1, 3, 4, 10, and 11). More satisfactory results were obtained using the octanuclear complex 4 (entry 2) and 7 a, which led to 8 a in an excellent 97 % yield (entry 5). When air was replaced by pure O2 (2 bar), 8 a was formed quantitatively (entry 6). However, either no reaction or only traces of 8 a were observed under strict anaerobic conditions and also when CO was replaced by CO2 (entries 7 and 8). A lower yield was obtained by using toluene as the solvent (entry 9). As reported,31 the carbonylation in the presence of AgOAc only proceeded stoichiometrically (entries 12 and 13). Nanoparticles prepared by the procedure of Hutchison32 from different gold complexes, 1, 3, and 7 a, gave low yields of 8 b, even when relatively high catalyst loadings were used (entries 14–18). No urea was obtained with CuOAc, and also not with the gold(I) complexes [Au(tht)Cl], [AuCl(PPh3)], and [JohnPhosAu(NCMe)](SbF6)33 under the standard reaction conditions.
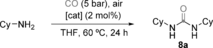
Entry |
Catalyst |
Variation from the standard reaction conditions |
Yield [%][b] |
|---|---|---|---|
1 |
3 |
– |
12 |
2 |
4 |
– |
50 |
3 |
6 a |
– |
30 |
4 |
6 b |
– |
13 |
5 |
7 a |
– |
97[c] |
6 |
7 a |
O2 (2 bar) |
99 |
7 |
7 a |
anaerobic |
0 |
8 |
7 a |
CO2 instead CO |
<5 |
9 |
7 a |
toluene |
20 |
10 |
7 b |
– |
35 |
11 |
7 c |
– |
22 |
12 |
AgOAc |
– |
<5 |
13 |
AgOAc |
20 mol % |
20 |
14 |
NP1 |
– |
3 |
15 |
NP1 |
20 mol % |
21 |
16 |
NP2 |
10 mol % |
<1 |
17 |
NP3 |
– |
7 |
18 |
NP3 |
20 mol % |
37 |
- [a] Standard reaction conditions: Heating at 60 °C for CyNH2, catalyst (2 mol %), CO (5 bar), air, 24 h. [b] Yields determined by 1H NMR spectroscopy with Ph3CH as an internal standard. [c] Yield of the isolated product. NP=nanoparticles prepared from 1 (NP1), 3 (NP2), 6 a (NP3). THF=tetrahydrofuran.
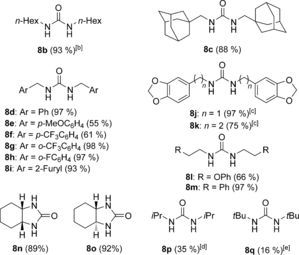
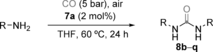
Under the optimized reaction conditions using 7 a as the catalyst, the carbonylation reaction of aliphatic amines gave rise to the ureas 8 b–o in good to excellent yields, although when more sterically hindered iPrNH2 and tBuNH2 were used, the corresponding products 8 p and 8 q were obtained in modest yields (lower temperatures had to be used because of the low boiling point of these amines; Table 2). In the case of n-HexNH2, the urea 8 b was obtained at room temperature in excellent yield. For the less reactive primary amines such as adamantylamine, the formation of the intermediate isocyanate was observed by GC-MS.34 Secondary amines such as (nPr)2NH are unreactive under the optimized reaction conditions, which is consistent with the involvement of isocyanates as intermediates. Accordingly, under the reaction conditions of the oxidative carbonylation, mixtures of nBuNH2 and (nPr)2NH gave di-n-butylurea and nBuNH(C=O)N(n-Pr)2 with 7 a as the catalyst, but none of the symmetrical urea (nPr)2N(C=O)N(nPr)2 was formed.34 N-cyclohexylformamide was unreactive under the oxidative carbonylation in the presence of 7 a.34 We did not observe any induction period when monitoring the formation of 8 b, from n-hexylamine over time, by 1H NMR spectroscopy when using 7 a as the catalyst,34 thus suggesting that the reaction occurs under homogeneous conditions.
|
- [a] Reaction at 60 °C, catalyst (2 mol %), 24 h. Yield is that of the isolated product determined as an average of 2 runs. [b] Reaction at 25 °C. [c] 2 bars of O2 were used instead of air. [d] 30 °C. [e] 40 °C.
Other experiments were performed to understand the nature of the catalyst using the carbonylation of n-hexylamine as a model system.35 Thus, when the reaction mixture was filtered after 45 minutes through a HPLC filter (PTFE 0.2 μm) and Celite, 8 b was still obtained in 86 % yield.34 After addition of amine at the end of the carbonylation, the reaction restarted, thus forming 8 b again (73 % yield). We also isolated a small amount of insoluble black solid in the filtration of the reaction mixture, and it did not dissolve in the presence of an amine and also did not show any catalytic activity. Although the mechanism for the formation of isocyanates and ureas under the oxidative carbonylation reaction is not totally clear, in contrast to that observed by the group of Bertrand,7 no reaction of either 3, 4, or 7 a with CO (5 bar) was observed even after 24 hours. Interestingly, formation of a new species was detected upon reaction of 7 a with CyNH2, and it showed a single signal in the 31P NMR spectrum at δ=31 ppm and a cluster of peaks at m/z 2455–2462 (MALDI-TOF) which corresponds to the quasi-molecular ion of pentanuclear complex [L2Au4Ag(NHCy)2](SbF6)3 (Figure 1).
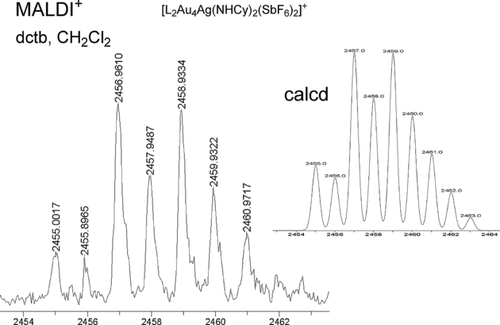
MALDI-TOF spectrum of 7 a + CyNH2. L=(Ph2P)2Py.
In summary, we have developed a simple synthesis of a linear tetracationic tetranuclear [AuI4] complex in one step from [Au2Cl2{(Ph2P)2Py}], and it induces C−H functionalization of carbonyl compounds under mild reaction conditions. We have also prepared a pentanuclear [AuI4AgI] complex by reaction of [AuI4] with AgI, which is highly catalytically active for the oxidative carbonylation of primary amines to form ureas under homogeneous conditions, with efficiencies comparable to other transition-metal-catalyzed processes and higher than those achieved by gold nanoparticles. We have also obtained an octanuclear complex with an unprecedented and almost planar [AuI8] core, and it is formally the product of a 4+4 reaction of two [Au4] complexes. Further studies on the synthesis of other complexes along these lines and further mechanistic studies are under way.
Acknowledgements
We thank MINECO [Severo Ochoa Excellence Accreditation 2014-2018 (SEV-2013-0319) CTQ2013-42106-P and CTQ2014-52824-R], the European Research Council (Advanced Grant No. 321066), the AGAUR (2014 SGR 818), and the ICIQ Foundation. N.A.G.B. gratefully acknowledges COFUND/Marie Curie action 291787-ICIQ-IPMP for funding. We also thank the ICIQ X-ray diffraction unit for the X-ray structures.