Regulating the Rate of Molecular Self-Assembly for Targeting Cancer Cells
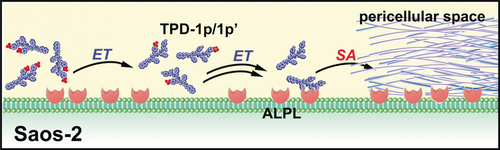
Graphical Abstract
Tailoring the number of phosphates on a peptidic substrate enables regulation of the rate of self-assembly of the enzyme reaction product. Such a rate regulation allows selective inhibition of osteosarcoma cells over hepatocytes, which constitutes a promising approach to target cancer cells in a specific organ.
Abstract
Besides tight and specific ligand–receptor interactions, the rate regulation of the formation of molecular assemblies is one of fundamental features of cells. But the latter receives little exploration for developing anticancer therapeutics. Here we show that a simple molecular design of the substrates of phosphatases—tailoring the number of phosphates on peptidic substrates—is able to regulate the rate of molecular self-assembly of the enzyme reaction product. Such a rate regulation allows selective inhibition of osteosarcoma cells over hepatocytes, which promises to target cancer cells in a specific organ. Moreover, our result reveals that the direct measurement of the rate of the self-assembly in a cell-based assay provides precise assessment of the cell targeting capability of self-assembly. This work, as the first report establishing rate regulation of a multiple-step process to inhibit cells selectively, illustrates a fundamentally new approach for controlling the fate of cells.