Hydrophosphination of CO2 and Subsequent Formate Transfer in the 1,3,2-Diazaphospholene-Catalyzed N-Formylation of Amines
Che Chang Chong
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Rei Kinjo
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21, Singapore 637371 (Singapore)Search for more papers by this authorChe Chang Chong
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Rei Kinjo
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Nanyang Link 21, Singapore 637371 (Singapore)Search for more papers by this authorGraphical Abstract
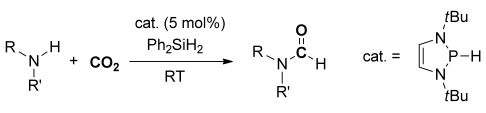
Formate formation: Hydrophosphination of CO2 with 2-H-1,3,2-diazaphospholene afforded phosphorus formate, from which transfer of the formate to Ph2SiH2 produced Ph2Si(OCHO)2. These elementary reactions were applied to the metal-free catalytic N-formylation of various amine derivatives with CO2 in a one-pot approach at room temperature.
Abstract
Hydrophosphination of CO2 with 1,3,2-Diazaphospholene (NHP-H; 1) afforded phosphorus formate (NHP-OCOH; 2) through the formation of a bond between the electrophilic phosphorus atom in 1 and the oxygen atom from CO2, along with hydride transfer to the carbon atom of CO2. Transfer of the formate from 2 to Ph2SiH2 produced Ph2Si(OCHO)2 (3) in a reaction that could be carried out in a catalytic manner by using 5 mol % of 1. These elementary reactions were applied to the metal-free catalytic N-formylation of amine derivatives with CO2 in one pot under ambient conditions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201505244_sm_miscellaneous_information.pdf2.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. M. D’Alessandro, B. Smit, J. R. Long, Angew. Chem. Int. Ed. 2010, 49, 6058–6082; Angew. Chem. 2010, 122, 6194–6219;
- 1bM. Z. Jacobson, Energy Environ. Sci. 2009, 2, 148–173;
- 1cG. Olah, A. Goeppert, G. K. Surya Prakash, J. Org. Chem. 2009, 74, 487–498;
- 1d Carbon Dioxide Capture and Storage (Eds.: ), Intergovernmental Panel on Climate Change (IPCC), Cambridge University Press, New York, 2005.
- 2
- 2aQ. Liu, L. Wu, R. Jackstell, M. Beller, Nat. Commun. 2015, 6, 5933;
- 2bA. Tlili, E. Blondiaux, X. Frogneux, T. Cantat, Green Chem. 2015, 17, 157–168;
- 2cC. S. Yeung, V. M. Dong, Top. Catal. 2014, 57, 1342–1350;
- 2dC. Maeda, Y. Miyazaki, T. Ema, Catal. Sci. Technol. 2014, 4, 1482–1497;
- 2eA. M. Appel, J. E. Bercaw, A. B. Bocarsly, H. Dobbek, D. L. DuBois, M. Dupuis, J. G. Ferry, E. Fujita, R. Hille, P. J. A. Kenis, C. A. Kerfeld, R. H. Morris, C. H. F. Peden, A. R. Portis, S. W. Ragsdale, T. B. Rauchfuss, J. N. H. Reek, L. C. Seefeldt, R. K. Thauer, G. L. Waldrop, Chem. Rev. 2013, 113, 6621–6658;
- 2fS. N. Riduan, Y. Zhang, Dalton Trans. 2010, 39, 3347–3357;
- 2gT. Sakakura, J. Choi, H. Yasuda, Chem. Rev. 2007, 107, 2365–2387.
- 3Selected recent examples and references therein:
- 3aN. M. Rezayee, C. A. Huff, M. S. Sanford, J. Am. Chem. Soc. 2015, 137, 1028–1031;
- 3bH. Suh, L. M. Guard, N. Hazari, Chem. Sci. 2014, 5, 3859–3872;
- 3cS. Moret, P. J. Dyson, G. Laurenczy, Nat. Commun. 2014, 5, 4017;
- 3dS. Bontemps, L. Vendier, S. Sabo-Etienne, J. Am. Chem. Soc. 2014, 136, 4419–4425;
- 3eA. Berkefeld, W. E. Piers, M. Parvez, L. Castro, L. Maron, O. Eisenstein, Chem. Sci. 2013, 4, 2152–2162;
- 3fS. Bontemps, S. Sabo-Etienne, Angew. Chem. Int. Ed. 2013, 52, 10253–10255; Angew. Chem. 2013, 125, 10443–10445;
- 3gR. Dobrovetsky, D. W. Stephan, Angew. Chem. Int. Ed. 2013, 52, 2516–2519; Angew. Chem. 2013, 125, 2576–2579; See also:
- 3hC. Lescot, D. U. Nielsen, I. S. Makarov, A. T. Lindhardt, K. Daasbjerg, T. Skrydstrup, J. Am. Chem. Soc. 2014, 136, 6142–6147.
- 4F. Fontaine, M. Courtemanche, M. Légaré, Chem. Eur. J. 2014, 20, 2990–2996.
- 5
- 5aD. Martin, M. Soleilhavoup, G. Bertrand, Chem. Sci. 2011, 2, 389–399;
- 5bP. P. Power, Nature 2010, 463, 171–177;
- 5cD. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2010, 49, 46–76; Angew. Chem. 2010, 122, 50–81.
- 6C. M. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2009, 48, 6643–6646; Angew. Chem. 2009, 121, 6770–6773.
- 7D. W. Stephan, G. Erker, Angew. Chem. Int. Ed. 2015, 54, 6400–6441; Angew. Chem. 2015, 127, 6498–6541.
- 8Selected recent examples:
- 8aM. Courtemanche, A. P. Pulis, É. Rochette, M. Légaré, D. W. Stephan, F. Fontaine, Chem. Commun. 2015, 51, 9797–9800;
- 8bG. Ménard, T. M. Gilbert, J. A. Hatnean, A. Kraft, I. Krossing, D. W. Stephan, Organometallics 2013, 32, 4416–4422;
- 8cG. Ménard, D. W. Stephan, Angew. Chem. Int. Ed. 2011, 50, 8396–8399; Angew. Chem. 2011, 123, 8546–8549;
- 8dX. Zhao, D. W. Stephan, Chem. Commun. 2011, 47, 1833–1835;
- 8eD. A. Dickie, E. N. Coker, R. A. Kemp, Inorg. Chem. 2011, 50, 11288–11290;
- 8fG. Ménard, D. W. Stephan, J. Am. Chem. Soc. 2010, 132, 1796–1797; Selected examples of the catalytic transformation of CO2:
- 8gT. Wang, D. W. Stephan, Chem. Eur. J. 2014, 20, 3036–3039;
- 8hT. Wang, D. W. Stephan, Chem. Commun. 2014, 50, 7007–7010;
- 8iC. Das Neves Gomes, E. Blondiaux, P. Thuéry, T. Cantat, Chem. Eur. J. 2014, 20, 7098–7106;
- 8jM. Courtemanche, M. Légaré, L. Maron, F. Fontaine, J. Am. Chem. Soc. 2013, 135, 9326–9329;
- 8kY. Wang, Y. Wang, W. Zhang, X. Lu, J. Am. Chem. Soc. 2013, 135, 11996–12003;
- 8lR. Wehmschulte, M. Saleh, D. R. Powell, Organometallics 2013, 32, 6812–6819;
- 8mM. Courtemanche, J. Larouche, M. Légaré, W. Bi, L. Maron, F. Fontaine, Organometallics 2013, 32, 6804–6811;
- 8nA. Schäfer, W. Saak, D. Haase, T. Müller, Angew. Chem. Int. Ed. 2012, 51, 2981–2984; Angew. Chem. 2012, 124, 3035–3038;
- 8oM. Khandelwal, R. J. Wehmschulte, Angew. Chem. Int. Ed. 2012, 51, 7323–7326; Angew. Chem. 2012, 124, 7435–7439;
- 8pS. N. Riduan, Y. Zhang, J. Y. Ying, Angew. Chem. Int. Ed. 2009, 48, 3322–3325; Angew. Chem. 2009, 121, 3372–3375;
- 8qY. Kayaki, M. Yamamoto, T. Ikariya, Angew. Chem. Int. Ed. 2009, 48, 4194–4197; Angew. Chem. 2009, 121, 4258–4261.
- 9
- 9aL. J. Murphy, K. N. Robertson, R. A. Kemp, H. M. Tuononen, J. A. C. Clyburne, Chem. Commun. 2015, 51, 3942–3956;
- 9bI. Purushothaman, S. De, P. Parameswaran, RSC Adv. 2014, 4, 60421–60428.
- 10aC.-C. Chong, R. Kinjo, ACS Catal. 2015, 5, 3238–3259;
- 10bR. Declercq, G. Bouhadir, D. Bourissou, M. Légaré, M. Courtemanche, K. S. Nahi, N. Bouchard, F. Fontaine, L. Maron, ACS Catal. 2015, 5, 2513–2520;
- 10cM. A. Courtemanche, M. A. Légaré, L. Maron, F. Fontaine, J. Am. Chem. Soc. 2014, 136, 10708–10717; See also:
- 10dC. Lim, A. M Holder, J. T. Hynes, C. B. Musgrave, Inorg. Chem. 2013, 52, 10062–10066.
- 11
- 11aI. Knopf, C. C. Cummins, Organometallics 2015, 34, 1601–1603;
- 11bZ. Lu, H. Hausmann, S. Becker, H. A. Wagner, J. Am. Chem. Soc. 2015, 137, 5332–5335;
- 11cK. Fujiwara, S. Yasuda, T. Mizuta, Organometallics 2014, 33, 6692–6695;
- 11dI. Peuser, R. C. Neu, X. Zhao, M. Ulrich, B. Schirmer, J. A. Tannert, G. Kehr, R. Fröhlich, S. Grimme, G. Erker, D. W. Stephan, Chem. Eur. J. 2011, 17, 9640–9650;
- 11eA. Berkefeld, W. E. Piers, M. Parvez, J. Am. Chem. Soc. 2010, 132, 10660–10661;
- 11fA. E. Ashley, A. L. Thompson, D. O’Hare, Angew. Chem. Int. Ed. 2009, 48, 9839–9843; Angew. Chem. 2009, 121, 10023–10027;
- 11gT. Wartik, R. K. Pearson, J. Inorg. Nucl. Chem. 1958, 7, 404–411;
- 11hT. Wartik, R. K. Pearson, J. Am. Chem. Soc. 1955, 77, 1075.
- 12
- 12aG. Ménard, D. W. Stephan, Dalton Trans. 2013, 42, 5447–5453;
- 12bP. Kuo, I. Chen, J. Chang, M. Lee, C. Hu, C. Hung, H. M. Lee, J. Huang, Eur. J. Inorg. Chem. 2004, 4898–4906.
- 13J. A. B. Abdalla, I. M. Riddlestone, R. Tirfoin, S. Aldridge, Angew. Chem. Int. Ed. 2015, 54, 5098–5102; Angew. Chem. 2015, 127, 5187–5191.
- 14
- 14aA. Jana, G. Tavčar, H. W. Roesky, M. John, Dalton Trans. 2010, 39, 9487–9489;
- 14bA. Jana, D. Ghoshal, H. W. Roesky, I. Objartel, G. Schwab, D. Stalke, J. Am. Chem. Soc. 2009, 131, 1288–1293; For Si, see:
- 14cP. Arya, J. Boyer, R. J. P. Corriu, G. F. Lanneau, M. Perrot, J. Organomet. Chem. 1988, 346, C 11–C14;
- 14dM. Mehta, M. H. Holthausen, I. Mallov, M. Pérez, Z. Qu, S. Grimme, D. W. Stephan, Angew. Chem. Int. Ed. 2015, 54, 8250–8254; Angew. Chem. 2015, 127, 8368–8372.
- 15
- 15aA. Jana, H. W. Roesky, C. Schulzke, A. Döring, Angew. Chem. Int. Ed. 2009, 48, 1106–1109; Angew. Chem. 2009, 121, 1126–1129;
- 15bG. Albertin, S. Antoniutti, J. Castro, S. García-Fontán, G. Zanardo, Organometallics 2007, 26, 2918–2930.
- 16
- 16aR. G. Cavell, K. I. The, L. V. Griend, Inorg. Chem. 1981, 20, 3813–3818;
- 16bK. I. The, L. V. Griend, W. A. Whitla, R. G. Cavell, J. Am. Chem. Soc. 1977, 99, 7379–7380.
- 17L. J. Hounjet, C. B. Caputo, D. W. Stephan, Angew. Chem. Int. Ed. 2012, 51, 4714–4717; Angew. Chem. 2012, 124, 4792–4795.
- 18M. Courtemanche, M. Légaré, É. Rochette, F. Fontaine, Chem. Commun. 2015, 51, 6858–6861.
- 19
- 19aB. Chatelet, L. Joucla, J. Dutasta, A. Martinez, K. C. Szeto, V. Dufaud, J. Am. Chem. Soc. 2013, 135, 5348–5351;
- 19bR. W. Light, L. D. Hutchins, R. T. Paine, C. F. Campana, Inorg. Chem. 1980, 19, 3597–3604;
- 19cG. Oertel, H. Malz, H. Holtschmidt, Chem. Ber. 1964, 97, 891–902.
- 20For relevant examples, see:
- 20aD. R. Kindra, I. J. Casely, M. E. Fieser, J. W. Ziller, F. Furche, W. J. Evans, J. Am. Chem. Soc. 2013, 135, 7777–7787;
- 20bS. Yin, J. Maruyama, T. Yamashita, S. Shimada, Angew. Chem. Int. Ed. 2008, 47, 6590–6593; Angew. Chem. 2008, 120, 6692–6695.
- 21
- 21aD. Gudat, Acc. Chem. Res. 2010, 43, 1307–1316;
- 21bS. Burck, D. Gudat, M. Nieger, W. W. D. Mont, J. Am. Chem. Soc. 2006, 128, 3946–3955;
- 21cD. Gudat, A. Haghverdi, M. Nieger, Angew. Chem. Int. Ed. 2000, 39, 3084–3086;
10.1002/1521-3773(20000901)39:17<3084::AID-ANIE3084>3.0.CO;2-R CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 3211–3214.
- 22
- 22aC.-C. Chong, H. Hirao, R. Kinjo, Angew. Chem. Int. Ed. 2015, 54, 190–194; Angew. Chem. 2015, 127, 192–196;
- 22bC.-C. Chong, H. Hirao, R. Kinjo, Angew. Chem. Int. Ed. 2014, 53, 3342–3346; Angew. Chem. 2014, 126, 3410–3414.
- 23
- 23aC. Das Neves Gomes, O. Jacquet, C. Villiers, P. Thuéry, M. Ephritikhine, T. Cantat, Angew. Chem. Int. Ed. 2012, 51, 187–190; Angew. Chem. 2012, 124, 191–194;
- 23bO. Jacquet, C. D. N. Gomes, M. Ephritikhine, T. Cantat, J. Am. Chem. Soc. 2012, 134, 2934–2937; For a recent example with metal catalyst, see:
- 23cL. Zhang, Z. Han, X. Zhao, Z. Wang, K. Ding, Angew. Chem. Int. Ed. 2015, 54, 6186–6189; Angew. Chem. 2015, 127, 6284–6287.
- 24
- 24aE. Blondiaux, J. Pouessel, T. Cantat, Angew. Chem. Int. Ed. 2014, 53, 12186–12190; Angew. Chem. 2014, 126, 12382–12386;
- 24bS. Das, F. D. Bobbink, G. Laurenczy, P. J. Dyson, Angew. Chem. Int. Ed. 2014, 53, 12876–12879; Angew. Chem. 2014, 126, 13090–13093.
- 25
- 25aB. Wang, Z. Cao, RSC Adv. 2013, 3, 14007–14015; See also:
- 25bL. González-Sebastián, M. Flores-Alamo, J. J. García, Organometallics 2013, 32, 7186–7194;
- 25cS. Itagaki, K. Yamaguchi, N. Mizuno, J. Mol. Catal. A 2013, 366, 347–352;
- 25dR. Shintani, K. Nozaki, Organometallics 2013, 32, 2459–2462;
- 25eW. Sattler, G. Parkin, J. Am. Chem. Soc. 2012, 134, 17462–17465.
- 26
- 26aO. Jacquet, X. Frogneux, C. D. N. Gomes, T. Cantat, Chem. Sci. 2013, 4, 2127–2131;
- 26bI. Sorribes, K. Junge, M. Beller, Chem. Eur. J. 2014, 20, 7878–7883; See also:
- 26cL. González-Sebastián, M. Flores-Alamo, J. J. García, Organometallics 2015, 34, 763–769.