Synthesis of Bridged Diketopiperazines by Using the Persistent Radical Effect and a Formal Synthesis of Bicyclomycin
Tynchtyk Amatov
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic)
Search for more papers by this authorDr. Radek Pohl
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic)
Search for more papers by this authorDr. Ivana Císařová
Department of Inorganic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 2030/8, 12843 Prague (Czech Republic)
Search for more papers by this authorCorresponding Author
Dr. Ullrich Jahn
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic)
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic)Search for more papers by this authorTynchtyk Amatov
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic)
Search for more papers by this authorDr. Radek Pohl
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic)
Search for more papers by this authorDr. Ivana Císařová
Department of Inorganic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 2030/8, 12843 Prague (Czech Republic)
Search for more papers by this authorCorresponding Author
Dr. Ullrich Jahn
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic)
Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, Flemingovo náměstí 2, 16610 Prague (Czech Republic)Search for more papers by this authorGraphical Abstract
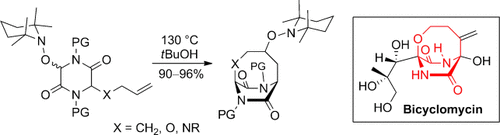
Persistent cyclization: A unified radical approach to diverse bridged diketopiperazines was developed by taking advantage of the persistent radical effect. The method allows rapid access to three-dimensional heterocyclic architectures and was applied to a formal synthesis of the antibiotic bicyclomycin.
Abstract
A conceptually new and unified approach to diverse bridged diketopiperazines (DKPs) with widely variable ring sizes was developed by taking advantage of the persistent radical effect. This method enables synthesis of the core structures of bridged DKP alkaloids and was applied to a formal synthesis of the antibiotic bicyclomycin.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201504883_sm_miscellaneous_information.pdf9.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1B. Over, S. Wetzel, C. Grütter, Y. Nakai, S. Renner, D. Rauh, H. Waldmann, Nat. Chem. 2013, 5, 21–28.
- 2
- 2aT. Amatov, U. Jahn, Angew. Chem. Int. Ed. 2014, 53, 3312–3314; Angew. Chem. 2014, 126, 3378–3380;
- 2bA. D. Borthwick, Chem. Rev. 2012, 112, 3641–3716;
- 2cJ. F. González, I. Ortín, E. de La Cuesta, J. C. Menéndez, Chem. Soc. Rev. 2012, 41, 6902–6915.
- 3
- 3aA. Arcelli, D. Balducci, G. Porzi, M. Sandri, Chem. Biodiversity 2010, 7, 225–228;
- 3bF. Piccinelli, G. Porzi, M. Sandri, S. Sandri, Tetrahedron: Asymmetry 2003, 14, 393–398.
- 4
- 4aM. Weigl, B. Wünsch, Org. Lett. 2000, 2, 1177–1179;
- 4bS. K. Sunnam, D. Schepmann, B. Wibbeling, B. Wünsch, Org. Biomol. Chem. 2010, 8, 3715–3722;
- 4cC. Geiger, C. Zelenka, K. Lehmkuhl, D. Schepmann, W. Englberger, B. Wünsch, J. Med. Chem. 2010, 53, 4212–4222 and references therein.
- 5Reviews on synthetic approaches to diaza[2.2.2]bicyclooctanes and natural products containing this core structure:
- 5aK. A. Miller, R. M. Williams, Chem. Soc. Rev. 2009, 38, 3160–3174;
- 5bC. F. Nising, Chem. Soc. Rev. 2010, 39, 591–599.
- 6More recent approaches to bicyclo[2.2.2]diazaoctane ring systems not covered in the review:
- 6aF. C. Frebault, N. S. Simpkins, Tetrahedron 2010, 66, 6585–6596;
- 6bF. C. Frebault, N. S. Simpkins, A. Fenwick, J. Am. Chem. Soc. 2009, 131, 4214–4215;
- 6cP. J. Crick, N. S. Simpkins, A. Highton, Org. Lett. 2011, 13, 6472–6475;
- 6dN. Simpkins, I. Pavlakos, L. Male, Chem. Commun. 2012, 48, 1958–1960;
- 6eN. S. Simpkins, I. Pavlakos, M. D. Weller, L. Male, Org. Biomol. Chem. 2013, 11, 4957–4970;
- 6fE. N. Morris, E. K. Nenninger, R. D. Pike, J. R. Scheerer, Org. Lett. 2011, 13, 4430–4433;
- 6gK. A. Margrey, A. J. Chinn, S. W. Laws, R. D. Pike, J. R. Scheerer, Org. Lett. 2012, 14, 2458–2461;
- 6hS. W. Laws, J. R. Scheerer, J. Org. Chem. 2013, 78, 2422–2429;
- 6iK. A. Margrey, A. D. Hazzard, J. R. Scheerer, Org. Lett. 2014, 16, 904–907;
- 6jA. Cabanillas, C. D. Davies, L. Male, N. S. Simpkins, Chem. Sci. 2015, 6, 1350–1354.
- 7
- 7aR. M. Williams, M. R. Sabol, H.-D. Kim, A. Kwast, J. Am. Chem. Soc. 1991, 113, 6621–6633;
- 7bR. M. Williams, A. Kwast, J. Org. Chem. 1988, 53, 5785–5787;
- 7cR. M. Williams, L. K. Maruyama, J. Org. Chem. 1987, 52, 4044–4047.
- 8A review of approaches to bicyclomycin and analogues: R. M. Williams, C. A. Durham, Chem. Rev. 1988, 88, 511–540.
- 9Two examples of making bridged DKPs by using ring-closing metathesis:
- 9aP. Besada, L. Mamedova, C. J. Thomas, S. Costanzi, K. A. Jacobson, Org. Biomol. Chem. 2005, 3, 2016–2025;
- 9bM. Pichowicz, N. S. Simpkins, A. J. Blake, C. Wilson, Tetrahedron 2008, 64, 3713–3735.
- 10S. B. Herzon, A. G. Myers, J. Am. Chem. Soc. 2005, 127, 5342–5344.
- 11
- 11aB. M. Trost, N. Cramer, H. Bernsmann, J. Am. Chem. Soc. 2007, 129, 3086–3087;
- 11bB. M. Trost, D. A. Bringley, T. Zhang, N. Cramer, J. Am. Chem. Soc. 2013, 135, 16720–16735.
- 12
- 12aP. S. Baran, C. A. Guerrero, N. B. Ambhaikar, B. D. Hafensteiner, Angew. Chem. Int. Ed. 2005, 44, 606–609; Angew. Chem. 2005, 117, 612–615;
- 12bP. S. Baran, C. A. Guerrero, B. D. Hafensteiner, N. B. Ambhaikar, Angew. Chem. Int. Ed. 2005, 44, 3892–3895; Angew. Chem. 2005, 117, 3960–3963;
- 12cP. S. Baran, B. D. Hafensteiner, N. B. Ambhaikar, C. A. Guerrero, J. D. Gallagher, J. Am. Chem. Soc. 2006, 128, 8678–8693.
- 13Reviews on the PRE:
- 13aH. Fischer, Chem. Rev. 2001, 101, 3581–3610;
- 13bA. Studer, Chem. Eur. J. 2001, 7, 1159–1164;
10.1002/1521-3765(20010316)7:6<1159::AID-CHEM1159>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 13cA. Studer, Chem. Soc. Rev. 2004, 33, 267–273;
- 13dA. Studer, T. Schulte, Chem. Rec. 2005, 5, 27–35.
- 14L. Tebben, A. Studer, Angew. Chem. Int. Ed. 2011, 50, 5034–5068; Angew. Chem. 2011, 123, 5138–5174.
- 15
- 15aA. Studer, Angew. Chem. Int. Ed. 2000, 39, 1108–1111;
10.1002/(SICI)1521-3773(20000317)39:6<1108::AID-ANIE1108>3.0.CO;2-A CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 1157–1160;
- 15bC. Wetter, K. Jantos, K. Woithe, A. Studer, Org. Lett. 2003, 5, 2899–2902;
- 15cA. Teichert, K. Jantos, K. Harms, A. Studer, Org. Lett. 2004, 6, 3477–3480;
- 15dY. Uenoyama, M. Tsukida, T. Doi, I. Ryu, A. Studer, Org. Lett. 2005, 7, 2985–2988;
- 15eB. Janza, A. Studer, Org. Lett. 2006, 8, 1875–1878;
- 15fC. Wetter, A. Studer, Chem. Commun. 2004, 174–175;
- 15gK. Molawi, T. Schulte, K. O. Siegenthaler, C. Wetter, A. Studer, Chem. Eur. J. 2005, 11, 2335–2350;
- 15hA. J. Herrera, A. Studer, Synthesis 2005, 1389–1396; see also:
- 15iC. Leroi, B. Fenet, J.-L. Couturier, O. Guerret, M. A. Ciufolini, Org. Lett. 2003, 5, 1079–1081;
- 15jC. Leroi, D. Bertin, P.-E. Dufils, D. Gigmes, S. Marque, P. Tordo, J.-L. Couturier, O. Guerret, M. A. Ciufolini, Org. Lett. 2003, 5, 4943–4945;
- 15kD. Bertin, D. Gigmes, S. R. A. Marque, P. Tordo, Tetrahedron 2005, 61, 8752–8761.
- 16
- 16aJ. Xu, E. J. E. Caro-Diaz, L. Trzoss, E. A. Theodorakis, J. Am. Chem. Soc. 2012, 134, 5072–5075;
- 16bJ. Xu, E. J. E. Caro-Diaz, M. H. Lacoske, C.-I. Hung, C. Jamora, E. A. Theodorakis, Chem. Sci. 2012, 3, 3378–3386.
- 17
- 17aA. W. Hung, A. Ramek, Y. Wang, T. Kaya, J. A. Wilson, P. A. Clemons, D. W. Young, Proc. Natl. Acad. Sci. USA 2011, 108, 6799–6804;
- 17bF. Lovering, J. Bikker, C. Humblet, J. Med. Chem. 2009, 52, 6752–6756.
- 18A similar thermal homolysis to give captodative sialic acid derived radicals: P. K. Kancharla, T. Kato, D. Crich, J. Am. Chem. Soc. 2014, 136, 5472–5480.
- 19
- 19aU. Jahn, J. Org. Chem. 1998, 63, 7130–7131;
- 19bE. Dinca, P. Hartmann, J. Smrček, I. Dix, P. G. Jones, U. Jahn, Eur. J. Org. Chem. 2012, 4461–4482.
- 20Products 14 d and 14 e were obtained as an inseparable mixture of four diastereomers, which was simplified by oxidative deprotection of the 2,2,6,6-tetramethylpiperidin-1-yl group at the exocyclic stereocenter to a ketone. See the Supporting Information for details.
- 21Leading references on 8-endo-trig cyclizations in nonbridged systems:
- 21aK. Ghosh, A. K. Ghosh, U. R. Ghatak, J. Chem. Soc. Chem. Commun. 1994, 629–630;
- 21bX. Fang, K. Liu, C. Li, J. Am. Chem. Soc. 2010, 132, 2274–2283.
- 22Examples of 9-endo-trig cyclizations in nonbridged systems:
- 22aK. Ghosh, U. R. Ghatak, Tetrahedron Lett. 1995, 36, 4897–4900;
- 22bS. E. Gibson, N. Guillo, M. J. Tozer, Chem. Commun. 1997, 637–638;
- 22cL. Song, K. Liu, C. Li, Org. Lett. 2011, 13, 3434–3437.
- 23J. Kuang, S. Ma, J. Org. Chem. 2009, 74, 1763–1765.
- 24M. Hayashi, M. Shibuya, Y. Iwabuchi, Org. Lett. 2012, 14, 154–157.
- 25Leading reference for radical cyclizations onto allenes: J. Shi, M. Zhang, Y. Fu, L. Liu, Q.-X. Guo, Tetrahedron 2007, 63, 12681–12688.
- 26M. Movassaghi, O. K. Ahmad, J. Org. Chem. 2007, 72, 1838–1841.
- 27
- 27aR. M. Williams, R. W. Armstrong, J.-S. Dung, J. Am. Chem. Soc. 1984, 106, 5748–5750;
- 27bR. M. Williams, R. W. Armstrong, J.-S. Dung, J. Am. Chem. Soc. 1985, 107, 3253–3266.