Participation of Alkoxy Groups in Reactions of Acetals: Violation of the Reactivity/Selectivity Principle in a Curtin–Hammett Kinetic Scenario†
This research was supported by the National Institutes of Health, National Institute of General Medical Sciences (GM-61066). We thank Olga Lavinda for assistance with computational studies, Dr. Chin Lin for help with NMR spectroscopy and mass spectrometric data, and Dr. Chunhua Hu for his assistance with crystallographic structure determination.
Graphical Abstract
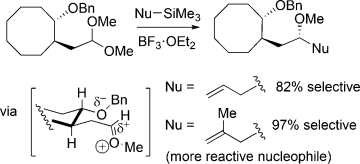
On principle: Nucleophilic substitution reactions of acetals having benzyloxy groups four carbon atoms away can be highly diastereoselective. The selectivity in several cases increased as the reactivity of the nucleophile increased, in violation of the reactivity/selectivity principle. The increase in selectivity with reactivity suggests that multiple conformational isomers of reactive intermediates can give rise to the products.
Abstract
Nucleophilic substitution reactions of acetals having benzyloxy groups four carbon atoms away can be highly diastereoselective. The selectivity in several cases increased as the reactivity of the nucleophile increased, in violation of the reactivity/selectivity principle. The increase in selectivity with reactivity suggests that multiple conformational isomers of reactive intermediates can give rise to the products.