[HCo(CO)4]-Catalyzed Three-component Cycloaddition of Epoxides, Imines, and Carbon Monoxide: Facile Construction of 1,3-Oxazinan-4-ones†
Lixia Liu
State Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, and Department of Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Huailin Sun
State Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, and Department of Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
State Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, and Department of Chemistry, Nankai University, Tianjin, 300071 (P.R. China)Search for more papers by this authorLixia Liu
State Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, and Department of Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
Search for more papers by this authorCorresponding Author
Prof. Huailin Sun
State Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, and Department of Chemistry, Nankai University, Tianjin, 300071 (P.R. China)
State Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, and Department of Chemistry, Nankai University, Tianjin, 300071 (P.R. China)Search for more papers by this authorThis work was supported by the NSFC (Project No. 20834002), the Natural Science Foundation of Tianjin (Project No. 08JCZDJC21600), and the Ministry of Education of China (Project No. 03406).
Graphical Abstract
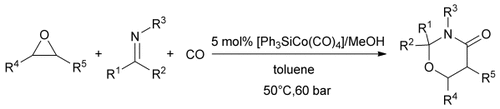
Cobalt and CO: The title reaction is described to proceed in the presence of [HCo(CO)4] as the catalyst. The reaction occurs for a wide variety of imines and various substituted epoxides, thus providing an efficient and atom-economic route to 1,3-oxazinan-4-ones, with various substitution patterns, from simple and readily available starting materials.
Abstract
The three-component [3+2+1] cycloaddition of epoxides, imines, and carbon monoxide to produce 1,3-oxazinan-4-ones has been developed by using [HCo(CO)4] as the catalyst. The reaction occurs for a wide variety of imines and epoxides, under 60 bar of CO pressure at 50 °C, to produce 1,3-oxazinan-4-ones with different substitution patterns in high yields, and provides an efficient and atom-economic route to heterocycles from simple and readily available starting materials. A plausible mechanism involves [HCo(CO)4]-induced ring-opening of the epoxide, followed by sequential addition of carbon monoxide and the imine, and then ring closure to form the product accompanied by regeneration of [HCo(CO)4].
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201403998_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For general reviews on transition-metal-catalyzed heterocycles synthesis, see:
- 1aX. Wu, H. Neumann, M. Beller, Chem. Rev. 2013, 113, 1;
- 1bA. V. Gulevich, A. S. Dudnik, N. Chernyak, V. Gevorgyan, Chem. Rev. 2013, 113, 3084;
- 1cI. Nakamura, Y. Yamamoto, Chem. Rev. 2004, 104, 2127;
- 1dN. T. Patil, Y. Yamamoto, Chem. Rev. 2008, 108, 3395.
- 2For synthesis of heterocycles by multicomponent reactions, see:
- 2aJ. S. Quesnel, B. A. Arndtsen, Pure Appl. Chem. 2013, 85, 377;
- 2bB. A. Arndtsen, Chem. Eur. J. 2009, 15, 302;
- 2cI. Marek, Tetrahedron 2005, 61, 11299.
- 3For synthesis of heterocycles by cycloaddition reactions, see:
- 3aS. Perreault, T. Rovis, Chem. Soc. Rev. 2009, 38, 3149;
- 3bN. Weding, M. Hapke, Chem. Soc. Rev. 2011, 40, 4525.
- 4
- 4aP. R. Chopade, J. Louie, Adv. Synth. Catal. 2006, 348, 2307;
- 4bN. Chatani, Chem. Rec. 2008, 8, 201;
- 4cB. Heller, M. Hapke, Chem. Soc. Rev. 2007, 36, 1085;
- 4dJ. A. Varela, C. Saá, Chem. Rev. 2003, 103, 3787.
- 5aN. Chatani, M. Tobisu, T. Asaumi, S. Murai, J. Am. Chem. Soc. 1999, 121, 7160;
- 5bM. Tobisu, N. Chatani, T. Asaumi, S. Murai, J. Am. Chem. Soc. 2000, 122, 12663;
- 5cN. Chatani, K. Amako, M. Tobisu, S. Murai, J. Org. Chem. 2003, 68, 1591.
- 6aN. Chatani, M. Tobisu, T. Asaumi, S. Murai, Synthesis 2000, 925;
- 6bT. Kondo, M. Nomura, Y. Ura, J. Am. Chem. Soc. 2006, 128, 14816;
- 6cP. Mathur, R. K. Joshi, D. K. Rai, Dalton Trans. 2012, 41, 5045;
- 6dT. Ozawa, H. Horie, T. Kurahashi, S. Matsubara, Chem. Commun. 2010, 46, 8055.
- 7aT. Fukuyama, Y. Higashibeppu, R. Yamaura, I. Ryu, Org. Lett. 2007, 9, 587;
- 7bB. Wu, R. Hua, Tetrahedron Lett. 2010, 51, 6433.
- 8aH. Hoberg, G. Burkhart, Synthesis 1979, 525;
- 8bH. Bönnemann, Angew. Chem. 1985, 97, 264;
10.1002/ange.19850970406 Google ScholarAngew. Chem. Int. Ed. Engl. 1985, 24, 248;
- 8cT. Takahashi, F. Y. Tsai, K. Nakajima, J. Am. Chem. Soc. 2002, 124, 5059;
- 8dM. M. McCormick, H. A. Duong, J. Louie, J. Am. Chem. Soc. 2005, 127, 5030.
- 9
- 9aH. A. Duong, J. Louie, J. Organomet. Chem. 2005, 690, 5098;
- 9bT. Kondo, M. Nomura, Y. Ura, Tetrahedron Lett. 2006, 47, 7107;
- 9cK. M. Oberg, E. E. Lee, T. Rovis, Tetrahedron 2009, 65, 5056.
- 10
- 10aR. Dhawan, R. D. Dghaym, B. A. Arndtsen, J. Am. Chem. Soc. 2003, 125, 1474;
- 10bR. Dhawan, B. A. Arndtsen, J. Am. Chem. Soc. 2004, 126, 468;
- 10cR. Dhawan, R. D. Dghaym, B. A. Arndtsen, Org. Lett. 2006, 8, 3927.
- 11A. R. Siamaki, B. A. Arndtsen, J. Am. Chem. Soc. 2006, 128, 6050.
- 12
- 12aR. D. Dghaym, R. Dhawan, B. A. Arndtsen, Angew. Chem. 2001, 113, 3328;
10.1002/1521-3757(20010903)113:17<3328::AID-ANGE3328>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3228;10.1002/1521-3773(20010903)40:17<3228::AID-ANIE3228>3.0.CO;2-Q CAS PubMed Web of Science® Google Scholar
- 12bS. Bontemps, J. S. Quesnel, K. Worrall, B. A. Arndtsen, Angew. Chem. 2011, 123, 9110;
10.1002/ange.201103885 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8948;
- 12cK. Worrall, B. Xu, S. Bontemps, B. A. Arndtsen, J. Org. Chem. 2011, 76, 170;
- 12dB. Xu, K. Worrall, B. A. Arndtsen, Molecules 2012, 17, 13759.
- 13A. R. Siamaki, D. A. Black, B. A. Arndtsen, J. Org. Chem. 2008, 73, 1135.
- 14
- 14aC. Larksarp, H. Alper, J. Org. Chem. 1999, 64, 9194;
- 14bC. Larksarp, H. Alper, J. Org. Chem. 2000, 65, 2773.
- 15J. Salvadori, E. Balducci, S. Zaza, E. Petricci, J. Org. Chem. 2010, 75, 1841.
- 16H. A. Duong, J. Louie, Tetrahedron 2006, 62, 7552.
- 17T. Miura, M. Morimoto, M. Murakami, J. Am. Chem. Soc. 2010, 132, 15836.
- 18T. L. Church, C. M. Byrne, E. B. Lobkovsky, G. W. Coates, J. Am. Chem. Soc. 2007, 129, 8156.
- 19
- 19aF. Berhal, S. Takechi, N. Kumagai, M. Shibasaki, Chem. Eur. J. 2011, 17, 1915;
- 19bT. Ginman, Y. Gravenfors, J. Med. Chem. 2013, 56, 4181.
- 20
- 20aG. Cardillo, M. A. Hashem, C. Tomasini, J. Chem. Soc. Perkin. Trans. 1 1990, 1487;
- 20bE. Bandini, G. Martelli, G. Spunta, A. Bongini, M. Panunzio, Synlett 1999, 1735.
- 21M. Panunzio, E. Bandini, E. Campana, P. Vicennati, Tetrahedron: Asymmetry 2002, 13, 2113.
- 22
- 22aD. Ntirampebura, L. Ghosez, Tetrahedron Lett. 1999, 40, 7079;
- 22bD. Ntirampebura, L. Ghosez, Synthesis 2002, 2043.
- 23M. Panunzio, A. Bongini, M. Monari, E. Tamanini, E. Bandini, Tetrahedron 2004, 60, 8347.
- 24
- 24aM. Panunzio, E. Tamanini, P. Vicennati, Tetrahedron 2006, 62, 12270;
- 24bM. Panunzio, E. Bandini, Synthesis 2008, 1753.
- 25
- 25aT. Kametani, K. Kigasawa, M. Hiiragi, Heterocycles 1977, 7, 919;
- 25bT. Kametani, K. Kigasawa, M. Hiiragi, Heterocycles 1978, 9, 819;
- 25cF. Fülöp, G. Bernáth, K. Pihlaja, Adv. Heterocycl. Chem. 1997, 69, 349;
- 25dA. N. Cayley, K. A. Gallagher, R. J. K. Taylor, Synthesis 2008, 3846.
- 26
- 26aM. Panunzio, E. Bandini, A. Millemaggi, Synthesis 2007, 2060;
- 26bY. Watanabe, T. Washio, J. Krishnamurthi, M. Anada, S. Hashimoto, Chem. Commun. 2012, 48, 6969.
- 27H. Sun, J. Zhang, Q. Liu, L. Yu, J. Zhao, Angew. Chem. 2007, 119, 6180–6184; Angew. Chem. Int. Ed. 2007, 46, 6068–6072.
- 28L. Jia, Polym. Prepr. 2010, 51, 406.
- 29Y. Zhang, H. Sun, Chem. J. Chin. Univ. 2014, 35, 54.
- 30C. M. Byrne, T. L. Church, J. W. Kramer, G. W. Coates, Angew. Chem. 2008, 120, 4043–4047;
10.1002/ange.200705310 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3979–3983.
- 31
- 31aR. F. Heck, J. Am. Chem. Soc. 1963, 85, 1460;
- 31bJ. Kreisz, F. Ungváry, A. Sisak, L. Markó, J. Organomet. Chem. 1991, 417, 89.
- 32
- 32aH. Alper, H. Arzoumanian, J. F. Petrignani, M. S. Maldonado, J. Chem. Soc. Chem. Commun. 1985, 340;
- 32bT. Murai, E. Yasui, S. Murai, J. Am. Chem. Soc. 1989, 111, 7938.
- 33J. Kreisz, A. Sisak, L. Markó, F. Ungváry, J. Organomet. Chem. 1993, 451, 53.
- 34
- 34aH. Böhme, K. Hartke, Chem. Ber. 1963, 96, 600;
- 34bV. V. Vintonyak, M. Calà, F. Lay, B. Kunze, F. Sasse, M. E. Maier, Chem. Eur. J. 2008, 14, 3709.
- 35After preparation of the manuscript, a report appeared on an identical reaction using [Co2(CO)8]/LiCl as the catalyst. We thank the referee for reminding us of the important information. For details, see: Y. Zhang, J. Ji, X. Zhang, S. Lin, Q. Pan, L. Jia, Org. Lett. 2014, 16, 2130.