Covalent Attachment of Porphyrins and Ferrocenes to Electrode Surfaces through Direct Anodic Oxidation of Terminal Ethynyl Groups†
Matthew V. Sheridan
Department of Chemistry, University of Vermont, Burlington, Vermont 05405 (USA)
Search for more papers by this authorDr. Kevin Lam
Department of Chemistry, School of Science and Technology Nazarbayev University, Astana, 010000 (Republic of Kazakhstan)
Search for more papers by this authorCorresponding Author
Prof. William E. Geiger
Department of Chemistry, University of Vermont, Burlington, Vermont 05405 (USA)
Department of Chemistry, University of Vermont, Burlington, Vermont 05405 (USA)===Search for more papers by this authorMatthew V. Sheridan
Department of Chemistry, University of Vermont, Burlington, Vermont 05405 (USA)
Search for more papers by this authorDr. Kevin Lam
Department of Chemistry, School of Science and Technology Nazarbayev University, Astana, 010000 (Republic of Kazakhstan)
Search for more papers by this authorCorresponding Author
Prof. William E. Geiger
Department of Chemistry, University of Vermont, Burlington, Vermont 05405 (USA)
Department of Chemistry, University of Vermont, Burlington, Vermont 05405 (USA)===Search for more papers by this authorWe are grateful to the National Science Foundation (CHE-1212339) for support of this research.
Graphical Abstract
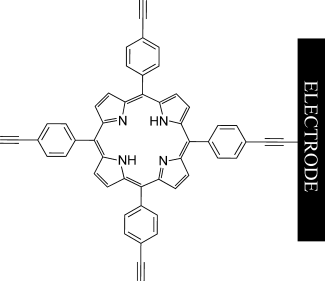
One with the surface: A method is presented for electrode modification with terminal alkynes and alkenes. Direct oxidation of these moieties leads to efficient grafting onto glassy carbon, gold, platinum, and indium tin oxide surfaces. Various ferrocenes and 5,10,15,20-(4-ethynylphenyl)porphyrin were attached in this way.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201307453_sm_miscellaneous_information.pdf465.5 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. Lissel, T. Fox, O. Blacque, W. Polit, R. F. Winter, K. Venkatesan, H. Berke, J. Am. Chem. Soc. 2013, 135, 4051–4060;
- 1bF. Gendron, A. Burgun, B. W. Skelton, A. H. White, T. Roisnel, M. I. Bruce, J.-F. Halet, C. Lapinte, K. Costuas, Organometallics 2012, 31, 6796–6811;
- 1cH. N. Roberts, N. J. Brown, R. Edge, E. C. Fitzgerald, Y. T. Ta, D. Collison, P. J. Low, M. W. Whiteley, Organometallics 2012, 31, 6322–6335;
- 1dS. Szafert, J. A. Gladysz, Chem. Rev. 2006, 106, PR 1–PR33;
- 1eT. Ren, Organometallics 2005, 24, 4854–4870.
- 2M. V. Sheridan, K. Lam, W. E. Geiger, J. Am. Chem. Soc. 2013, 135, 2939–2942.
- 3 Acetylene Chemistry (Eds.: ), Wiley-VCH, Weinheim, 2005.
- 4D. Bélanger, J. Pinson, Chem. Soc. Rev. 2011, 40, 3995–4048.
- 5P. Aschwanden, E. M. Carreira, Acetylene Chemistry (Eds.: ), Wiley-VCH, Weinheim, 2005, p. 101.
- 6A second oxidation at the iron center (P. R. Sharp, A. J. Bard, Inorg. Chem. 1983, 22, 2689–2693) is likely to come about 2 V positive of E1/2Ox1.
- 7The oxidation potentials of alkynes are typically 1 V vs. ferrocene or slightly higher. See
- 7aY. Fu, L. Liu, H.-Z. Yu, Y.-M. Wang, Q.-X. Guo, J. Am. Chem. Soc. 2005, 127, 7227–7234;
- 7bM. Katz, H. Wendt, Electrochim. Acta 1976, 21, 215–218;
- 7cR. Bauer, H. Wendt, J. Electroanal. Chem. 1977, 80, 395–399.
- 8Multiple scans through the second wave in the deposition solution resulted in irreproducible coverage and disfigured surface waves for 1; the surface coverage for an idealized monolayer of ferrocene is 4.5×10−10 mol cm−2. See K. Seo, I. C. Jeon, D. J. Yoo, Langmuir 2004, 20, 4147–4154.
- 9
- 9aS. Drouet, S. Ballut, J. Rault-Berthelot, P. Turban, C. Paul-Roth, Thin Solid Films 2009, 517, 5474–5481;
- 9bC. Paul-Roth, J. Rault-Berthelot, G. Simonneaux, C. Poriel, M. Abdalilah, J. Letessier, J. Electroanal. Chem. 2006, 597, 19–27;
- 9cJ. Rault-Berthelot, C. Paul-Roth, C. Poriel, S. Juillard, S. Ballut, S. Drouet, G. Simmoneaux, J. Electroanal. Chem. 2008, 623, 204–214.
- 10
- 10aJ. E. Huthchinson, T. A. Postlethwaite, C.-H. Chen, K. W. Hathcock, R. S. Ingram, W. Ou, R. W. Linton, R. W. Murrary, D. A. Tyvoll, L. L. Chng, J. P. Collman, Langmuir 1997, 13, 2143–2148;
- 10bH. H. De Paz, C. Médard, M. Morin, J. Electroanal. Chem. 2010, 648, 163–168.
- 11S. Ssenyange, F. Anariba, D. F. Bocian, R. L. McCreery, Langmuir 2005, 21, 11105–11112.
- 12
- 12aA. R. McDonald, N. Franssen, G. P. M. van Klink, G. van Koten, J. Organomet. Chem. 2009, 694, 2153–2162;
- 12bP. K. B. Palomaki, P. H. Dinolfo, Langmuir 2010, 26, 9677–9685.
- 13
- 13aM. Picot, I. Nicolas, C. Poriel, J. Rault-Berthelot, F. Barrière, Electrochem. Commun. 2012, 20, 167–170;
- 13bA. J. Gross, C. Bucher, L. Coche-Guerente, P. Labbé, A. J. Downard, J.-C. Moutet, Electrochem. Commun. 2011, 13, 1236–1239.
- 14K. M. Kadish, E. Van Caemelbecke, J. Solid State Electrochem. 2003, 7, 254–258.
- 15Compound 3 also has two reversible reductions in DCM/0.1 M [Bu4N][PF6] at −1.62 V, −1.95 V vs. FcH.
- 16D. A. Van Galen, M. Majda, Anal. Chem. 1988, 60, 1549–1553.
- 17
- 17aD.-J. Qian, C. Nakamura, T. Ishida, S.-O. Wenk, T. Wakayama, S. Takeda, J. Miyake, Langmuir 2002, 18, 10237–10242;
- 17bP. K. B. Palomaki, P. H. Dinoflo, ACS Appl. Mater. Interfaces 2011, 3, 4703–4713.
- 18
- 18aD. Clark, M. Fleischmann, D. Pletcher, J. Electroanal. Chem. 1972, 36, 137–146;
- 18bD. B. Clark, M. Fleischmann, D. Pletcher, J. Electroanal. Chem. 1973, 42, 133–138.