Highly Enantioselective Bromocyclization of Tryptamines and Its Application in the Synthesis of (−)-Chimonanthine†
Corresponding Author
Prof. Weiqing Xie
State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Weiqing Xie, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Yisheng Lai, State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)===
Dawei Ma, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Search for more papers by this authorGuangde Jiang
State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)
Search for more papers by this authorHuan Liu
State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)
Search for more papers by this authorJiadong Hu
State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)
Search for more papers by this authorXixian Pan
Department of Chemistry, School of Science, Shanghai University (China)
Search for more papers by this authorProf. Hui Zhang
Department of Chemistry, School of Science, Shanghai University (China)
Search for more papers by this authorXiaolong Wan
State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Yisheng Lai
State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)
Weiqing Xie, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Yisheng Lai, State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)===
Dawei Ma, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Search for more papers by this authorCorresponding Author
Prof. Dawei Ma
State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Weiqing Xie, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Yisheng Lai, State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)===
Dawei Ma, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Search for more papers by this authorCorresponding Author
Prof. Weiqing Xie
State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Weiqing Xie, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Yisheng Lai, State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)===
Dawei Ma, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Search for more papers by this authorGuangde Jiang
State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)
Search for more papers by this authorHuan Liu
State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)
Search for more papers by this authorJiadong Hu
State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)
Search for more papers by this authorXixian Pan
Department of Chemistry, School of Science, Shanghai University (China)
Search for more papers by this authorProf. Hui Zhang
Department of Chemistry, School of Science, Shanghai University (China)
Search for more papers by this authorXiaolong Wan
State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Search for more papers by this authorCorresponding Author
Prof. Yisheng Lai
State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)
Weiqing Xie, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Yisheng Lai, State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)===
Dawei Ma, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Search for more papers by this authorCorresponding Author
Prof. Dawei Ma
State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)
Weiqing Xie, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Yisheng Lai, State Key Laboratory of Natural Medicines, Center of Drug Discovery, China Pharmaceutical University, 24 Tongjiaxiang, Nanjing 210009 (China)===
Dawei Ma, State Key Laboratory of Bioorganic & Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032 (China)===
Search for more papers by this authorWe are grateful to the National Science Foundation of China (21202187, 21372239) for financial support.
Graphical Abstract
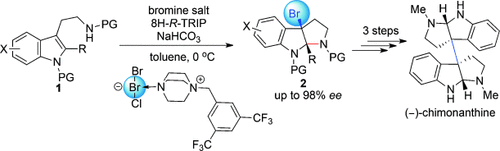
A shorter path: A highly enantioselective bromocyclization of tryptamine has been developed using an anionic chiral phase-transfer catalyst. This method provides a direct approach for preparing chiral 3-bromopyrroloindoline from tryptamine, which enables a four-step enantioselective synthesis of (−)-chimonanthine. PG=protecting group.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201306774_sm_miscellaneous_information.pdf7.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aU. Anthoni, C. Christophersen, P. H. Nielsen in Alkaloids: Chemical and Biological Perspectives, Vol. 13 (Ed.: ), Pergamon, New York, 1999, pp. 163–236;
10.1016/S0735-8210(99)80025-9 Google Scholar
- 1bG. A. Cordell, J. E. Saxton in The Alkaloids: Chemistry and Physiology, Vol. 20 (Eds.: ), Academic Press, New York, 1981, pp. 3–295;
- 1cT. Hino, M. Nakagawa in The Alkaloids: Chemistry and Pharmacology, Vol. 34 (Ed.: ), Academic Press, New York, 1989, pp. 1–75;
10.1016/S0099-9598(08)60226-6 Google Scholar
- 1dT. S. Pvenet, J. Pusset in The Alkaloids: Chemistry and Pharmacology, Vol. 48 (Ed.: ), Academic Press, New York, 1996, pp. 1–73.
- 2H. Takayama, I. Mori, M. Kitajima, N. Aimi, N. H. Lajis, Org. Lett. 2004, 6, 2945–2948.
- 3G. Massiot, P. Thépenier, M.-J. Jacquier, L. Men-Oliver, C. Delaude, Heterocycles 1989, 29, 1435–1438.
- 4R. H. Burnell, A. Chapelle, M. F. Khali, Can. J. Chem. 1974, 52, 2327–2330.
- 5For reviews, see:
- 5aP. Ruiz-Sanchis, S. A. Savina, F. Albericio, M. Alvarez, Chem. Eur. J. 2011, 17, 1388–1408;
- 5bA. Steven, L. E. Overman, Angew. Chem. 2007, 119, 5584–5605; Angew. Chem. Int. Ed. 2007, 46, 5488–5508;
- 5cM. A. Schmidt, M. Movassaghi, Synlett 2008, 313–324.
- 6For selected examples for enantioselective synthesis of chimonanthine and related alkaloids, see:
- 6aL. E. Overman, D. V. Paone, B. A. Stearns, J. Am. Chem. Soc. 1999, 121, 7702–7703;
- 6bL. E. Overman, J. F. Larrow, B. A. Stearns, J. M. Vance, Angew. Chem. 2000, 112, 219–221;
10.1002/(SICI)1521-3757(20000103)112:1<219::AID-ANGE219>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 2000, 39, 213–215;10.1002/(SICI)1521-3773(20000103)39:1<213::AID-ANIE213>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 6cM. Movassaghi, M. A. Schmidt, Angew. Chem. 2007, 119, 3799–3802;
10.1002/ange.200700705 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3725–3728;
- 6dC. Guo, J. Song, J. Z. Huang, P. H. Chen, S. W. Luo, L. Z. Gong, Angew. Chem. 2012, 124, 1070–1074; Angew. Chem. Int. Ed. 2012, 51, 1046–1050;
- 6eH. Mitsunuma, M. Shibasaki, M. Kanai, S. Matsunaga, Angew. Chem. 2012, 124, 5307–5311; Angew. Chem. Int. Ed. 2012, 51, 5217–5221;
- 6fR. R. Liu, J. L. Zhang, Org. Lett. 2013, 15, 2266–2269.
- 7
- 7aZ. Zuo, W. Xie, D. Ma, J. Am. Chem. Soc. 2010, 132, 13226–13228;
- 7bZ. Zuo, D. Ma, Angew. Chem. 2011, 123, 12214–12217; Angew. Chem. Int. Ed. 2011, 50, 12008–12011;
- 7cW. Zi, W. Xie, D. Ma, J. Am. Chem. Soc. 2012, 134, 9126–9129;
- 7dW. Xie, H. Wang, F. Fan, J. Tian, Z. Zuo, W. Zi, K. Gao, D. Ma, Tetrahedron Lett. 2013, 54, 4392–4396.
- 8For reviews, see:
- 8aA. Castellanos, S. P. Fletcher, Chem. Eur. J. 2011, 17, 5766–5776;
- 8bC. K. Tan, L. Zhou, Y.-Y. Yeung, Synlett 2011, 1335–1339; for halolactonization reactions, see:
- 8cD. C. Whitehead, R. Yousefi, A. Jaganathan, B. Borhan, J. Am. Chem. Soc. 2010, 132, 3298–3300;
- 8dW. Zhang, S. Zheng, N. Liu, J. B. Werness, I. A. Guzei, W. Tang, J. Am. Chem. Soc. 2010, 132, 3664–3665;
- 8eK. Murai, T. Matsushita, A. Nakamura, S. Fukushima, M. Shimura, H. Fujioka, Angew. Chem. 2010, 122, 9360–9363;
10.1002/ange.201005409 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 9174–9177;
- 8fG. E. Veitch, E. N. Jacobsen, Angew. Chem. 2010, 122, 7490–7493;
10.1002/ange.201003681 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7332–7335;
- 8gL. Zhou, C. K. Tan, X. Jiang, F. Chen, Y.-Y. Yeung, J. Am. Chem. Soc. 2010, 132, 15474–15476;
- 8hM. C. Dobish, J. N. Johnston, J. Am. Chem. Soc. 2012, 134, 6068–6071;
- 8iX. Jiang, C. K. Tan, L. Zhou, Y. Yueng, Angew. Chem. 2012, 124, 7891–7895; Angew. Chem. Int. Ed. 2012, 51, 7771–7775;
- 8jD. H. Paull, C. Fang, J. R. Donald, A. D. Pansick, S. F. Martin, J. Am. Chem. Soc. 2012, 134, 11128–11131; for other reactions, see:
- 8kS. E. Denmark, M. T. Burk, Org. Lett. 2012, 14, 256–259;
- 8lY. Zhang, H. Xing, W. Xie, X. Wan, Y. Lai, D. Ma, Adv. Synth. Catal. 2013, 355, 68–72;
- 8mF. Chen, C. K. Tan, Y. Y. Yeung, J. Am. Chem. Soc. 2013, 135, 1232–1235;
- 8nC. S. Brindle, C. S. Yeung, E. N. Jacobsen, Chem. Sci. 2013, 4, 2100–2104;
- 8oK. Mori, Y. Ichikawa, M. Kobayashi, Y. Shibata, M. Yamanaka, T. Akiyama, J. Am. Chem. Soc. 2013, 135, 3964–3970.
- 9
- 9aR. J. Phipps, G. L. Hamilton, F. D. Toste, Nat. Chem. 2012, 4, 603–614;
- 9bV. Rauniyar, A. D. Lackner, G. L. Hamilton, F. D. Toste, Science 2011, 334, 1681–1684;
- 9cR. J. Phipps, K. Hiramatsu, F. D. Toste, J. Am. Chem. Soc. 2012, 134, 8376–8379;
- 9dY.-M. Wang, J. Wu, C. Hoong, V. Rauniyar, F. D. Toste, J. Am. Chem. Soc. 2012, 134, 12928–12931;
- 9eT. Honjo, R. J. Phipps, V. Rauniyar, F. D. Toste, Angew. Chem. 2012, 124, 9822–9826;
10.1002/ange.201205383 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9684–9688;
- 9fR. J. Phipps, F. D. Toste, J. Am. Chem. Soc. 2013, 135, 1268–1271.
- 10For selected examples, see:
- 10aV. R. Espejo, J. D. Rainier, J. Am. Chem. Soc. 2008, 130, 12894–12895;
- 10bT. Newhouse, C. A. Lewis, K. J. Eastman, P. S. Baran, J. Am. Chem. Soc. 2010, 132, 7119–7137;
- 10cD. Tu, L. Ma, X. Tong, X. Deng, C. Xia, Org. Lett. 2012, 14, 4830–4833.
- 11
- 11aO. Lozano, G. Blessley, T. M. del Campo, A. L. Thompson, G. T. Giuffredi, M. Bettati, M. Walker, R. Borman, V. Gouverneur, Angew. Chem. 2011, 123, 8255–8259; Angew. Chem. Int. Ed. 2011, 50, 8105–8109;
- 11bYou and Cai have described that a similar reaction gave only moderate enatioselectivity using (DHQ)2PHAL as the catalyst; see: Q. Cai, Ph.D. thesis, Shanghai Institute of Orgnic Chemistry (CN), 2012, 87–100.
- 12
- 12aA. R. Hajipour, H. Imanieh, S. A. Pourmousavi, Synth. Commun. 2004, 34, 4597–4604;
- 12bF. Matloubi Moghaddam, H. Z. Boeini, Synlett 2005, 1612–1614.
- 13A benzyl or a methyl group on indole nitrogen caused the corresponding substrates to be halogenated on the aromatic ring instead of being cyclized to pyrroloindoline.
- 14CCDC 958615 (2 d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15An explanation of the observed stereochemical outcome has been proposed (see the Supporting Information).
- 16
- 16aM. Movassaghi, M. A. Schmidt, J. A. Ashehurst, Angew. Chem. 2008, 120, 1507–1509;
10.1002/ange.200704960 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1485–1487;
- 16bJ. Kim, J. A. Ashenhurst, M. Movassaghi, Science 2009, 324, 238–241;
- 16cJ. Kim, M. Movassaghi, J. Am. Chem. Soc. 2010, 133, 14376–14378;
- 16dM. Movassaghi, O. K. Ahmad, S. P. Lathrop, J. Am. Chem. Soc. 2011, 124, 13002–13005;
- 16eN. Boyer, M. Movassaghi, Chem. Sci. 2012, 3, 1798–1803.
- 17The improvement of the enantiopurity of dimer 3 could be ascribed to the difference in the reaction rate for homodimerization coupling and heterodimerization coupling of 2 a. The enantiomer of 2 a reacted preferentially with 2 a to form meso-3, which was removed by chromatography. Thus, the enantiopurity of the homodimerization product 3 was improved to 99 % by a resolution process.
- 18Y. Wang, C. Kong, Y. Du, H. Song, D. Zhang, Y. Qin, Org. Biomol. Chem. 2012, 10, 2793–2797.
- 19L. Furst, J. M. R. Narayanam, C. R. J. Stephenson, Angew. Chem. 2011, 123, 9829–9833;
10.1002/ange.201103145 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9655–9659.