Diastereoselective Synthesis of Eight-Membered-Ring Allenes from Propargylic Epoxides and Aldehydes by Silylene Insertion into Carbon–Oxygen Bonds†
Christina Z. Rotsides
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Search for more papers by this authorDr. Chunhua Hu
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Search for more papers by this authorCorresponding Author
Prof. K. A. Woerpel
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)===Search for more papers by this authorChristina Z. Rotsides
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Search for more papers by this authorDr. Chunhua Hu
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Search for more papers by this authorCorresponding Author
Prof. K. A. Woerpel
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)
Department of Chemistry, New York University, 100 Washington Square East, New York, NY 10003 (USA)===Search for more papers by this authorThe authors thank New York University and the Margaret and Herman Sokol Fellowship (to C.Z.R.) for support of this research. We thank the NYU Molecular Design Institute for the purchase of the Bruker SMART APEXII diffractometer.
Graphical Abstract
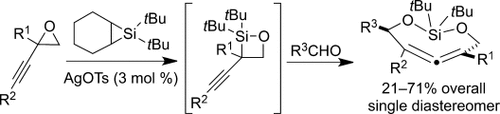
Bent out of shape: Silver-catalyzed insertions of silylenes into propargylic CO bonds of epoxides regioselectively form 1,2-silaoxetanes, which add to aldehydes to give the title allenes as single diastereomers (see scheme; Ts=4-toluenesulfonyl). An X-ray crystal structure confirmed the stereochemistry of the allene, which is bent significantly from linearity (164°).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201306093_sm_miscellaneous_information.pdf51.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. J. Daoust, S. M. Hernandez, K. M. Konrad, I. D. Mackie, J. Winstanley, R. P. Johnson, J. Org. Chem. 2006, 71, 5708–5714.
- 2D. Rodríguez, M. F. Martínez-Esperón, A. Navarro-Vázquez, L. Castedo, D. Domínguez, C. Saá, J. Org. Chem. 2004, 69, 3842–3848.
- 3T. Mahlokozera, J. B. Goods, A. M. Childs, D. M. Thamattoor, Org. Lett. 2009, 11, 5095–5097.
- 4J. D. Price, R. P. Johnson, J. Org. Chem. 1991, 56, 6372–6376.
- 5Larger nine- and ten-membered-ring allenes, however, which can accommodate the requisite allene geometry, are readily isolated. See, for example:
- 5aM. Ogasawara, A. Okada, K. Nakajima, T. Takahashi, Org. Lett. 2009, 11, 177–180;
- 5bC. Chiappe, A. De Rubertis, H. Detert, D. Lenoir, C. S. Wannere, P. von R. Schleyer, Chem. Eur. J. 2002, 8, 967–978;
10.1002/1521-3765(20020215)8:4<967::AID-CHEM967>3.0.CO;2-I CAS PubMed Web of Science® Google Scholar
- 5cM. Perscheid, D. Schollmeyer, U. Nubbemeyer, Eur. J. Org. Chem. 2011, 5250–5253;
- 5dJ. L. Luche, J. C. Damiano, C. Cohen-Addad, J. Am. Chem. Soc. 1980, 102, 5370–5374.
- 6X. Zhao, Z. Zhong, L. Peng, W. Zhang, J. Wang, Chem. Commun. 2009, 2535–2537.
- 7T. Shimizu, D. Miyasaka, N. Kamigata, J. Org. Chem. 2001, 66, 1787–1794.
- 8aC. M. Mömming, G. Kehr, B. Wibbeling, R. Fröhlich, B. Schirmer, S. Grimme, G. Erker, Angew. Chem. 2010, 122, 2464–2467;
10.1002/ange.200906697 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2414–2417.
- 9
- 9aB.-H. Xu, G. Kehr, R. Fröhlich, B. Wibbeling, B. Schirmer, S. Grimme, G. Erker, Angew. Chem. 2011, 123, 7321–7324; Angew. Chem. Int. Ed. 2011, 50, 7183–7186.
- 10T. Shimizu, F. Hojo, W. Ando, J. Am. Chem. Soc. 1993, 115, 3111–3115.
- 11Y. Pang, S. A. Petrich, V. G. Young, Jr., M. S. Gordon, T. J. Barton, J. Am. Chem. Soc. 1993, 115, 2534–2536.
- 12S. E. Gottschling, K. K. Milnes, M. C. Jennings, K. M. Baines, Organometallics 2005, 24, 3811–3814.
- 13M. Melaimi, M. Soleilhavoup, G. Bertrand, Angew. Chem. 2010, 122, 8992–9032;
10.1002/ange.201000165 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8810–8849.
- 14V. Lavallo, C. A. Dyker, B. Donnadieu, G. Bertrand, Angew. Chem. 2008, 120, 5491–5494;
10.1002/ange.200801176 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5411–5414.
- 15M. Melaimi, P. Parameswaran, B. Donnadieu, G. Frenking, G. Bertrand, Angew. Chem. 2009, 121, 4886–4889;
10.1002/ange.200901117 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4792–4795.
- 16F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, R. Taylor, J. Chem. Soc. Perkin Trans. 2 1987, S 1–S19.
- 17J. A. Marshall, Y. Tang, J. Org. Chem. 1993, 58, 3233–3234.
- 18J. A. Marshall, K. G. Pinney, J. Org. Chem. 1993, 58, 7180–7184.
- 19Aldehyde addition occurred in the absence of a silver catalyst. A control experiment in which the silver catalyst was sequestered using N,N-tetramethylethylenediamine prior to addition of the aldehyde demonstrated that silver is not required for aldehyde addition. For uncatalyzed allylation of carbonyl compounds using silacyclobutanes, see: K. Matsumoto, K. Oshima, K. Utimoto, J. Org. Chem. 1994, 59, 7152–7155.
- 20For examples of low reactivity of tetrasubstituted allenes, see:
- 20aC. Mukai, N. Kuroda, R. Ukon, R. Itoh, J. Org. Chem. 2005, 70, 6282–6290;
- 20bZ. Zhang, R. A. Widenhoefer, Org. Lett. 2008, 10, 2079–2081.
- 21For methods to remove the silylene protecting group to form diols, see: T. D. Nelson, R. D. Crouch, Synthesis 2000, 1031–1069.
- 22P. W. Dillon, G. R. Underwood, J. Am. Chem. Soc. 1974, 96, 779–787.
- 23The experimentally determined bond angle at the central carbon atom of the allene was slightly smaller than the 166.5° value calculated by ab initio methods (HF/6-311G*). Details are provided in the Supporting Information.
- 24A 1,3-bisthio eight-membered-ring allene, for example, contains a 162.50° bond angle, while its selenium counterpart contains a larger angle of 166.5°. See Refs. [6] and [7].
- 25The 13C NMR shifts of the central allene carbon did not change appreciably upon removal of the di-tert-butylsilyl group.
- 26J. Ye et al., Org. Lett. 2012, 14, 1346–1349, see the Supporting Information.
- 27M. Prévost, K. A. Woerpel, J. Am. Chem. Soc. 2009, 131, 14182–14183.
- 28Variable-temperature NMR spectroscopy experiments confirmed that 12 a and 12 b are distinct diastereomers rather than atropisomers.
- 29S. S. Kostina, W. J. Leigh, J. Am. Chem. Soc. 2011, 133, 4377–4388.
- 30It is also possible that zwitterionic intermediates are involved in this stepwise silylene insertion.
- 31L. E. Bourque, P. A. Cleary, K. A. Woerpel, J. Am. Chem. Soc. 2007, 129, 12602–12603.
- 32L. E. Bourque, P. A. Haile, J. M. N. Loy, K. A. Woerpel, Tetrahedron 2009, 65, 5608–5613.
- 33D. J. Henry, C. J. Parkinson, P. M. Mayer, L. Radom, J. Phys. Chem. A 2001, 105, 6750–6756.
- 34J. L. Wolk, T. Hoz, H. Basch, S. Hoz, J. Org. Chem. 2001, 66, 915–918.
- 35J. L. Wolk, M. Sprecher, H. Basch, S. Hoz, Org. Biomol. Chem. 2004, 2, 1065–1069.
- 36M. Prévost, J. W. Ziller, K. A. Woerpel, Dalton Trans. 2010, 39, 9275–9281.
- 37S. A. Sullivan, C. H. DePuy, R. Damrauer, J. Am. Chem. Soc. 1981, 103, 480–481.
- 38CCDC 950159 (8) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.