Fenestranes in Synthesis: Unique and Highly Inspiring Scaffolds
Correction(s) for this article
-
Corrigendum: Fenestranes in Synthesis: Unique and Highly Inspiring Scaffolds
- Volume 53Issue 4Angewandte Chemie International Edition
- pages: 911-911
- First Published online: January 21, 2014
Dr. Aicha Boudhar
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/
Search for more papers by this authorDr. Mélanie Charpenay
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/
Search for more papers by this authorDr. Gaëlle Blond
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/
Search for more papers by this authorCorresponding Author
Dr. Jean Suffert
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/===Search for more papers by this authorDr. Aicha Boudhar
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/
Search for more papers by this authorDr. Mélanie Charpenay
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/
Search for more papers by this authorDr. Gaëlle Blond
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/
Search for more papers by this authorCorresponding Author
Dr. Jean Suffert
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/
Faculté de Pharmacie, Université de Strasbourg, UMR 7200 CNRS/UDS, 74 Route du Rhin, Strasbourg (France) http://www-chimie.u-strasbg.fr/∼lsb/===Search for more papers by this authorGraphical Abstract
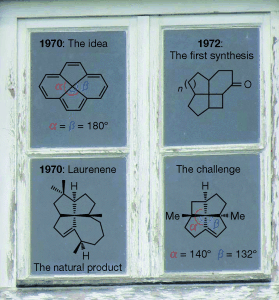
Highly strained: Four condensed cycles and a distorted tetracoordinated carbon center with bond angles greater than the regular 109.4° make the scaffold of fenestranes quite unique. A definition and nomenclature of these scaffolds is followed by a detailed overview over recent syntheses of these strained molecules, including their impact on the study of planar tetracoordinate carbon atoms.
Abstract
The scaffold of fenestranes is quite unique, as it contains four condensed cycles and a distorted tetracoordinated central carbon atom with bond angles greater than the regular 109°28“. In this Minireview, a detailed overview on the developments regarding this compound class, including their synthesis, is given for the time period since 2006. In the past years, natural products that belong to the class of heterofenestranes have been isolated and their syntheses will also be discussed.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304555_sm_miscellaneous_information.pdf1.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. H. van′t Hoff, Arch. Neerl. Sci. Exactes Nat. 1874, 9, 44;
- 1bJ. A. Le Bel, Bull. Soc. Chim. Fr. 1874, 22, 337.
- 2R. Hoffmann, R. W. Alder, C. F. Wilcox, Jr., J. Am. Chem. Soc. 1970, 92, 4992–4993.
- 3
- 3aK. B. Wiberg, G. B. Ellison, Tetrahedron 1974, 30, 1573–1578;
- 3bJ. B. Collins, J. D. Dill, E. D. Jemmis, Y. Apeloig, P. von R. Schleyer, R. Seeger, J. A. Pople, J. Am. Chem. Soc. 1976, 98, 5419–5427;
- 3cW. Siebert, A. Gunuale, Chem. Soc. Rev. 1999, 28, 367–371;
- 3dV. I. Minkin, R. M. Minyaev, R. Hoffmann, Russ. Chem. Rev. 2002, 71, 869–892.
- 4K. Sorger, P. von R. Schleyer, J. Mol. Struct. THEOCHEM 1995, 338, 317–346.
- 5For other examples of predicted structures, see:
- 5aP. von R. Schleyer, A. I. Boldyrev, J. Chem. Soc. Chem. Commun. 1991, 1536–1538;
- 5bL. S. Wang, A. I. Boldyrev, X. Li, J. Simons, J. Am. Chem. Soc. 2000, 122, 7681–7687;
- 5cX. Li, H.-J. Zhai, L.-S. Wang, Chem. Phys. Lett. 2002, 357, 415–419;
- 5dR. M. Minyaev, T. N. Gribanova, V. I. Minkin, A. G. Starikov, R. Hoffmann, J. Org. Chem. 2005, 70, 6693–6704;
- 5eT. N. Gribanova, R. M. Minyaev, V. I. Minkin, Russ. J. Gen. Chem. 2008, 78, 750–768; and references cited therein.
- 6For predicted planar C52− dianions, see:
- 6aR. W. Weber, J. M. Cook, Can. J. Chem. 1978, 56, 189–192;
- 6bG. Merino, M. A. Mendez-Rojas, A. Vela, J. Am. Chem. Soc. 2003, 125, 6026–6027;
- 6cA. J. Pihko, A. M. P. Koskinen, Tetrahedron 2005, 61, 8769–8807;
- 6dG. Merino, M. A. Mendez-Rojas, A. Vela, T. Heine, J. Comput. Chem. 2007, 28, 362–372.
- 7S. L. Buchwald, E. A. Lucas, W. M. Davis, J. Am. Chem. Soc. 1989, 111, 397–398.
- 8For other organometallic compounds, see Ref. [3c] and:
- 8aR. Choukroun, P. Cassoux, Acc. Chem. Res. 1999, 32, 494–502;
- 8bG. Erker, Chem. Soc. Rev. 1999, 28, 307–314;
- 8cM. Su, Inorg. Chem. 2005, 44, 4829–4833;
- 8dD. Roy, C. Corminboeuf, C. S. Wannere, R. B. King, P. von R. Schleyer, Inorg. Chem. 2006, 45, 8902–8906; and references cited therein.
- 9For caged hydrocarbon species, see:
- 9aJ. F. Liebman, A. Greenberg, Chem. Rev. 1976, 76, 311–365;
- 9bP. E. Eaton, B. D. Leipzig, J. Am. Chem. Soc. 1983, 105, 1656–1658;
- 9cK. B. Wiberg, Chem. Rev. 1989, 89, 975–983;
- 9dM. P. McGrath, L. Radom, H. F. Schaefer III, J. Org. Chem. 1992, 57, 4847–4850;
- 9eH. Dodziuk, J. Leszczyriski, K. S. Nowirinski, J. Org. Chem. 1995, 60, 6860–6863; and references cited therein.
- 10
- 10aL. Radom, D. R. Rasmussen, Pure Appl. Chem. 1998, 70, 1977–1984;
- 10bD. R. Rasmussen, L. Radom, Angew. Chem. 1999, 111, 3051–3054;
10.1002/(SICI)1521-3757(19991004)111:19<3051::AID-ANGE3051>3.0.CO;2-7 Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2875–2878.10.1002/(SICI)1521-3773(19991004)38:19<2875::AID-ANIE2875>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 11For other alkaplanes, see:
- 11aM. P. McGrath, L. Radom, J. Am. Chem. Soc. 1993, 115, 3320–3321;
- 11bD. R. Rasmussen, L. Radom, Chem. Eur. J. 2000, 6, 2470–2483.
10.1002/1521-3765(20000703)6:13<2470::AID-CHEM2470>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 12Z.-X. Wang, P. von R. Schleyer, J. Am. Chem. Soc. 2001, 123, 994–995.
- 13For planar species with higher coordination numbers, see:
- 13aK. Exner, P. von R. Schleyer, Science 2000, 290, 1937–1940;
- 13bY. Pei, X. C. Zeng, J. Am. Chem. Soc. 2008, 130, 2580–2592;
- 13cJ. O. C. Jimenez-Halla, Y. B. Wu, Z. X. Wang, R. Islas, T. Heine, G. Merino, Chem. Commun. 2010, 46, 8776–8778;
- 13dT. Heine, G. Merino, Angew. Chem. 2012, 124, 4349–4350;
10.1002/ange.201201166 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4275–4276.
- 14T. R. Galeev, C. Romanescu, W. L. Li, L. S. Wang, A. I. Boldyrev, Angew. Chem. 2012, 124, 2143–2147;
10.1002/ange.201107880 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2101–2105.
- 15V. Georgian, M. Saltzman, Tetrahedron Lett. 1972, 13, 4315–4317.
10.1016/S0040-4039(01)94304-7 Google Scholar
- 16R. Hoffmann, H. Hopf, Angew. Chem. 2008, 120, 4548–4556;
10.1002/ange.200705775 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4474–4481.
- 17Earlier contributions are resumed in the following reviews:
- 17aB. R. Venepalli, W. C. Agosta, Chem. Rev. 1987, 87, 399–410;
- 17bM. Thommen, R. Keese, Synlett 1997, 231–240;
- 17cR. Keese, Chem. Rev. 2006, 106, 4787–4808.
- 18J. F. Liebman, A. Greenberg, Chem. Rev. 1976, 76, 311–365.
- 19W. Luef, R. Keese, J. Mol. Struct. THEOCHEM 1992, 257, 353–366.
- 20A. K. Gupta, X. Fu, J. P. Snyder, J. M. Cook, Tetrahedron 1991, 47, 3665–3710.
- 21D. Hirschi, W. Luef, P. Gerber, R. Keese, Helv. Chim. Acta 1992, 75, 1897–1908.
- 22R. E. Corbett, D. R. Lauren, R. T. Weavers, J. Chem. Soc. Perkin Trans. 1 1979, 1774–1790.
- 23D. R. Lauren, Ph.D. Thesis, University of Otago, 1971.
- 24
- 24aR. E. Corbett, C. M. Couldwell, D. R. Lauren, R. T. Weavers, J. Chem. Soc. Perkin Trans. 1 1979, 1791–1794;
- 24bR. T. Weavers, J. Org. Chem. 2001, 66, 6453–6461.
- 25M. T. Crimmins, L. D. Gould, J. Am. Chem. Soc. 1987, 109, 6199–6200.
- 26T. Tsunoda, M. Amaike, U. S. F. Tambunan, Y. Fujise, S. Ito, M. Kodama, Tetrahedron Lett. 1987, 28, 2537–2540.
- 27P. A. Wender, T. W. Von Geldern, B. H. Levine, J. Am. Chem. Soc. 1988, 110, 4858–4860.
- 28For an alternative approach, see:
- 28aL. A. Paquette, M. E. Okazaki, J.-C. Caille, J. Org. Chem. 1988, 53, 477–481;
- 28bG. Mehta, K. S. Rao, J. Org. Chem. 1988, 53, 425–427.
- 29For reviews about angular triquinane natural products and their synthesis, see:
- 29aG. Mehta, A. Srikrishna, Chem. Rev. 1997, 97, 671–719;
- 29bL. A. Paquette, Top. Curr. Chem. 1979, 79, 41–165, particularly pages 108–133;
- 29cL. A. Paquette, Top. Curr. Chem. 1984, 119, 1–163;
- 29dL. A. Paquette, A. M. Doherty, Polyquinane Chemistry, Synthesis and Reactions, Springer, Berlin, 1987.
10.1007/978-3-642-72598-2 Google Scholar
- 30L. H. Zalkow, R. N. Harris III, N. I. Burke, J. Nat. Prod. 1979, 42, 96–102.
- 31For recent syntheses of isocomenes, see:
- 31aY. Iura, T. Sugahara, K. Ogasawara, Org. Lett. 2001, 3, 291–293;
- 31bA. W. Schmidt, T. Olpp, E. Baum, T. Stiffel, H.-J. Knölker, Synlett 2007, 15, 2371–2374;
- 31cA. W. Schmidt, T. Olpp, E. Baum, T. Stiffel, H.-J. Knölker, Org. Biomol. Chem. 2010, 8, 4562–4568.
- 32For syntheses of silphinanes, see:
- 32aF. Bohlmann, L. N. Misra, J. Javupovic, H. Robinson, R. M. King, J. Nat. Prod. 1984, 47, 658–662;
- 32bM. T. Crimmins, S. W. Mascarella, J. Am. Chem. Soc. 1986, 108, 3435–3438;
- 32cY. K. Rao, M. Nagarajan, J. Org. Chem. 1989, 54, 5678–5683;
- 32dN. T. Tzvetkov, T. Arndt, J. Mattay, Tetrahedron 2007, 63, 10497–10510; and references cited therein.
- 33For syntheses of silphiperfolanes, see:
- 33aA. S. Feliciano, J. M. M. Del Corral, E. Caballero, A. Alvarez, M. Medarde, J. Nat. Prod. 1986, 49, 845–853;
- 33bA. B. Trendafilova-Savkovaa, M. N. Todorovaa, C. V. Gussev, Z. Naturforsch. C 2003, 58, 817–819;
10.1515/znc-2003-11-1212 Google Scholar
- 33cA. Srikrishna, G. Nagaraju, V. M. Sheth, Tetrahedron 2012, 68, 2650–2656; and references cited therein.
- 34For syntheses of pentalenanes, see:
- 34aH. Seto, H. Yonehara, J. Antibiot. 1980, 33, 92–93;
- 34bG. Mehta, K. S. Rao, J. Am. Chem. Soc. 1986, 108, 8015–8021;
- 34cN. E. Schore, E. G. Rowley, J. Am. Chem. Soc. 1988, 110, 5224–5225;
- 34dN. M. Harrington-Frost, G. Pattenden, Tetrahedron Lett. 2000, 41, 403–405;
- 34eM. K. Pallerla, J. M. Fox, Org. Lett. 2007, 9, 5625–5628; and references cited therein.
- 35J. B. Gloer, M. R. TePaske, J. S. Sima, J. Org. Chem. 1988, 53, 5457–5460 and references cited therein. For a synthesis of aflavine, see: S. Danishefsky, S. Chackalamannil, P. Harrison, M. Silvestri, P. Cole, J. Am. Chem. Soc. 1985, 107, 2474–2484.
- 36M. Kladi, H. Xenaki, C. Vagias, P. Papazafiri, V. Roussis, Tetrahedron 2006, 62, 182–189.
- 37U. H. Brinker, T. Schrievers, L. Xu, J. Am. Chem. Soc. 1990, 112, 8609–8611.
- 38
- 38aFor an overview of syntheses of [m.n.p]fenestranes, see: Ref. [17a], pages 403–404;
- 38bfor a recent example, see: J. Deschamp, T. Hermant, O. Riant, Tetrahedron 2012, 68, 3457–3467.
- 39S. H. Shim, J. B. Gloer, D. T. Wicklow, J. Nat. Prod. 2006, 69, 1601–1605.
- 40S. H. Shim, D. C. Swenson, J. B. Gloer, P. F. Dowd, D. T. Wicklow, Org. Lett. 2006, 8, 1225–1228.
- 41T. Gaich, J. Mulzer, J. Am. Chem. Soc. 2009, 131, 452–453.
- 42T. Gaich, J. Mulzer, Org. Lett. 2010, 12, 272–275.
- 43N. Ingavat, C. Mahidol, S. Ruchirawat, P. Kittakoop, J. Nat. Prod. 2011, 74, 1650–1652.
- 44G. Mehta, T. B. Khan, Tetrahedron Lett. 2012, 53, 4558–4561.
- 45For a review of enone–olefin photocycloadditions, including syntheses of [m.n.p.q]fenestranes and [m.n.p]fenestranes, see: M. T. Crimmins, Chem. Rev. 1988, 88, 1453–1473.
- 46For some examples for syntheses of fenestranes through enone–olefin photocycloadditions, see the following publications and references cited therein:
- 46aW. G. Dauben, D. M. Walker, Tetrahedron Lett. 1982, 23, 711–714;
- 46bS. Wolff, B. R. Venepalli, C. F. George, W. C. Agosta, J. Am. Chem. Soc. 1988, 110, 6785–6790;
- 46cP. Gerber, R. Keese, Tetrahedron Lett. 1992, 33, 3987–3988.
- 47For a general review on the meta (or [3+2]) photocycloaddition of arenes to alkenes, see: J. Cornelisse, Chem. Rev. 1993, 93, 615–669.
- 48For some examples for syntheses of fenestranes through arene–olefin cycloadditions, see the following publications and references cited therein:
- 48aJ. Mani, S. Schuettel, C. Zhang, P. Bigler, C. Mueller, R. Keese, Helv. Chim. Acta 1989, 72, 487–495;
- 48bP. A. Wender, M. A. DeLong, F. C. Wireko, Acta Crystallogr. Sect C 1997, 53, 954–956.
- 49For some examples for syntheses of fenestranes through Weiss reactions, see: X. Fu, G. Kubiak, W. Zhang, W. Han, A. K. Gupta, J. M. Cook, Tetrahedron 1993, 49, 1511–1524 and references cited therein.
- 50For reviews by Kuck and co-workers on benzannelated fenestranes or other multiple fused cyclopentane and indane units, based on cyclodehydration and including fenestranes, see:
- 50aD. Kuck, Synlett 1996, 949–965;
- 50bD. Kuck in Advances in Theoretically Interesting Molecules, Vol. 4 (Ed.: ), JAI, Greenwich, London, 1998, p. 81–155;
10.1016/S1046-5766(98)80015-3 Google Scholar
- 50cD. Kuck, Chem. Rev. 2006, 106, 4885–4925.
- 51For some examples for syntheses of fenestranes through PKRs, see the following publications and references cited therein:
- 51aW. A. Smit, S. M. Bukhanyuk, S. O. Simonyan, A. S. Shashkov, Y. T. Struchkov, A. I. Yanovskii, R. Caple, A. S. Gybin, L. G. Anderson, J. A. Whiteford, Tetrahedron Lett. 1991, 32, 2105–2108;
- 51bA. Van der Waals, R. Keese, J. Chem. Soc. Chem. Commun. 1992, 570–571;
- 51cJ. Wang, R. Guidetti-Grept, R. Keese, H. Stoeckli-Evans, Helv. Chim. Acta 1997, 80, 1169–1175;
- 51dS. U. Son, K. H. Park, Y. K. Chung, J. Am. Chem. Soc. 2002, 124, 6838–6839.
- 52
- 52aS. E. Denmark, L. A. Kramps, J. I. Montgomery, Angew. Chem. 2002, 114, 4296–4299;
10.1002/1521-3757(20021104)114:21<4296::AID-ANGE4296>3.0.CO;2-4 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4122–4125;10.1002/1521-3773(20021104)41:21<4122::AID-ANIE4122>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 52bS. E. Denmark, J. I. Montgomery, Angew. Chem. 2005, 117, 3798–3802;
10.1002/ange.200500367 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3732–3736;
- 52cmechanistic study: R. L. Davis, D. J. Tantillo, J. Org. Chem. 2010, 75, 1693–1700.
- 53S. E. Denmark, J. I. Montgomery, L. A. Kramps, J. Am. Chem. Soc. 2006, 128, 11620–11630.
- 54C. S. Penkett, J. A. Woolford, I. J. Day, M. P. Coles, J. Am. Chem. Soc. 2010, 132, 4–5.
- 55C. S. Penkett, J. A. Woolford, T. W. Read, R. J. Kahan, J. Org. Chem. 2011, 76, 1295–1304.
- 56P. Macchi, W. Jing, R. Guidetti-Grept, R. Keese, Tetrahedron 2013, 69, 2479–2483.
- 57
- 57aR. Keese, Angew. Chem. 1992, 104, 307–309; Angew. Chem. Int. Ed. Engl. 1992, 31, 344–345;
- 57bD. Hirschi, W. Luef, P. Gerber, R. Keese, Helv. Chim. Acta 1992, 75, 1897–1908.
- 58
- 58aM. Thommen, M. Frötsch, R. Keese, Acta Crystallogr. Sect. C 1996, 52, 2051–2053;
- 58bM. Thommen, R. Keese, Synlett 1997, 231–240.
- 59M. Thommen, L. Prevot, M. K. Eberle, P. Bigler, R. Keese, Tetrahedron 2011, 67, 3868–3873.
- 60
- 60aP. Weyermann, R. Keese, Tetrahedron 2011, 67, 3874–3880;
- 60bP. Weyermann, R. Keese, H. Stoeckli-Evans, Acta Crystallogr. Sect. E 2010, 66, o 340.
- 61W. Chen, J.-H. Tay, J. Ying, X.-Q. Yu, L. Pu, J. Org. Chem. 2013, 78, 2256–2265.
- 62P. D. Thornton, D. J. Burnell, Org. Lett. 2006, 8, 3195–3198.
- 63C. Hulot, G. Blond, J. Suffert, J. Am. Chem. Soc. 2008, 130, 5046–5047.
- 64For the in silico study of the kinetics of these compounds, see: C. Hulot, S. Amiri, G. Blond, P. R. Schreiner, J. Suffert, J. Am. Chem. Soc. 2009, 131, 13387–13398.
- 65M. Charpenay, A. Boudhar, G. Blond, J. Suffert, Angew. Chem. 2012, 124, 4455–4458;
10.1002/ange.201107934 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4379–4382.
- 66For the study of a tandem reaction with 4-exo-dig cyclocarbopalladation and Sonogashira coupling, see: M. Charpenay, A. Boudhar, A. Siby, S. Schigand, G. Blond, J. Suffert, Adv. Synth. Catal. 2011, 353, 3151–3156.
- 67N. Heinrich, A. C. Willis, I. A. Cade, J. Ho, M. L. Coote, M. G. Banwell, Chem. Eur. J. 2012, 18, 13585–13588.
- 68
- 68aJ. E. Richman, H. E. Simmons, Tetrahedron 1974, 30, 1769–1774;
- 68bV. Galasso, D. Jones, J. E. Richman, J. Mol. Struct. THEOCHEM 1998, 429, 247–253.
- 69B. Ding, R. Keese, H. Stoeckli-Evans, Angew. Chem. 1999, 111, 387–388;
Angew. Chem. Int. Ed. 1999, 38, 375–376.
10.1002/(SICI)1521-3773(19990201)38:3<375::AID-ANIE375>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 70M. Ullrich, R. J. F. Berger, C. Lustig, R. Froehlich, N. W. Mitzel, Eur. J. Inorg. Chem. 2006, 4219–4224.
- 71B. R. Venepalli, C. F. George, S. Wolff, W. C. Agosta, J. Am. Chem. Soc. 1985, 107, 5732–5739.
- 72C. Hulot, J. Peluso, G. Blond, C. D. Muller, J. Suffert, Bioorg. Med. Chem. Lett. 2010, 20, 6836–6839.