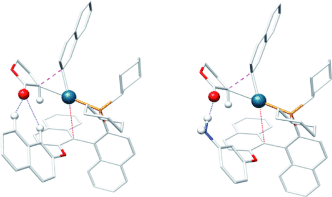
Weak Arene CH⋅⋅⋅O Hydrogen Bonding in Palladium-Catalyzed Arylation and Vinylation of Lactones†
Dr. Zhiyan Huang
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Zuliang Chen
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorLi Hui Lim
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorGia Chuong Phan Quang
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorProf. Dr. Hajime Hirao
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianrong (Steve) Zhou
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorDr. Zhiyan Huang
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorDr. Zuliang Chen
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorLi Hui Lim
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorGia Chuong Phan Quang
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorProf. Dr. Hajime Hirao
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Jianrong (Steve) Zhou
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, Singapore 637371 (Singapore)Search for more papers by this authorWe thank the Singapore National Research Foundation (NRF-RF2008-10) and Nanyang Technological University for financial support, and Johnson-Matthey for a gift of palladium.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201300481_sm_miscellaneous_information.pdf23.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews:
- 1aF. Bellina, R. Rossi, Chem. Rev. 2010, 110, 1082;
- 1bC. C. C. Johansson, T. J. Colacot, Angew. Chem. 2010, 122, 686;
10.1002/ange.200903424 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 676;
- 1cA. C. B. Burtoloso, Synlett 2009, 320;
- 1dJ. Zhou, Synlett 2012, 1;
- 1eD. A. Culkin, J. F. Hartwig, Acc. Chem. Res. 2003, 36, 234.
- 2
- 2aJ. Åhman, J. P. Wolfe, M. V. Troutman, M. Palucki, S. L. Buchwald, J. Am. Chem. Soc. 1998, 120, 1918;
- 2bX. Liao, Z. Weng, J. F. Hartwig, J. Am. Chem. Soc. 2008, 130, 195;
- 2cT. Hamada, A. Chieffi, J. Åhman, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 1261;
- 2dG. Chen, F. Y. Kwong, H. O. Chan, W.-Y. Yu, A. S. C. Chan, Chem. Commun. 2006, 1413;
- 2eS. Ge, J. F. Hartwig, J. Am. Chem. Soc. 2011, 133, 16330.
- 3
- 3aJ. García-Fortanet, S. L. Buchwald, Angew. Chem. 2008, 120, 8228;
10.1002/ange.200803809 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8108;
- 3bP. Nareddy, L. Mantilli, L. Guénée, C. Mazet, Angew. Chem. 2012, 124, 3892;
10.1002/ange.201108061 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3826.
- 4Examples:
- 4aA. M. Taylor, R. A. Altman, S. L. Buchwald, J. Am. Chem. Soc. 2009, 131, 9900;
- 4bS. Lee, J. F. Hartwig, J. Org. Chem. 2001, 66, 3402.
- 5D. J. Spielvogel, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 3500.
- 6
- 6aA. E. Allen, D. W. C. MacMillan, J. Am. Chem. Soc. 2011, 133, 4260;
- 6bJ. S. Harvey, S. P. Simonovich, C. R. Jamison, D. W. C. MacMillan, J. Am. Chem. Soc. 2011, 133, 13782;
- 6cA. Bigot, A. E. Williamson, M. J. Gaunt, J. Am. Chem. Soc. 2011, 133, 13778;
- 6dE. Skucas, D. W. C. MacMillan, J. Am. Chem. Soc. 2012, 134, 9090.
- 7Z. Huang, Z. Liu, J. Zhou, J. Am. Chem. Soc. 2011, 133, 15882.
- 8
- 8aE. Santaniello, P. Ferraboschi, P. Grisenti, A. Manzocchi, Chem. Rev. 1992, 92, 1071;
- 8bF. Theil, Chem. Rev. 1995, 95, 2203;
- 8cH. Pellissier, Tetrahedron 2003, 59, 8291;
- 8dT. Fischer, J. Pietruszka, Top. Curr. Chem. 2010, 297, 1, and references therein;
- 8eR. N. Patel, Coord. Chem. Rev. 2008, 252, 659, and references therein.
- 9
- 9aY. Chen, L. Deng, J. Am. Chem. Soc. 2001, 123, 11302;
- 9bC. Bolm, G. Schlingloff, K. Weickhardt, Angew. Chem. 1994, 106, 1944; Angew. Chem. Int. Ed. Engl. 1994, 33, 1848;
- 9cF. Hollmann, A. Taglieber, F. Schulz, M. T. Reetz, Angew. Chem. 2007, 119, 2961;
10.1002/ange.200605169 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 2903.
- 10
- 10aReview: J. T. Mohr, A. Y. Hong, B. M. Stoltz, Nat. Chem. 2009, 1, 359;
- 10bM. P. Carroll, H. Muller-Bunz, P. J. Guiry, Chem. Commun. 2012, 48, 11142;
- 10cC. H. Cheon, H. Yamamoto, J. Am. Chem. Soc. 2008, 130, 9246;
- 10dC. H. Cheon, O. Kanno, F. D. Toste, J. Am. Chem. Soc. 2011, 133, 13248;
- 10eA. Yanagisawa, T. Touge, T. Arai, Angew. Chem. 2005, 117, 1570;
10.1002/ange.200462325 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1546;
- 10fE. M. Beck, A. M. Hyde, E. N. Jacobsen, Org. Lett. 2011, 13, 4260.
- 11Ref. [2c].
- 12CCDC 919057 and 919058 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13
- 13aG. M. Sheldrick, SHELXL 93/97, Programs for Crystal Structure Refinement, University of Göttingen, Germany, 1993/1997. “…︁Generally speaking, a single phosphorus or heavier atom suffices to determine an absolute structure using Cu-K(alpha)radiation, and with accurate high-resolution low-temperature data including Friedel opposites such an atom may even suffice for Mo-K(alpha)”;
- 13bH. D. Flack, G. Bernardinelli, Chirality 2008, 20, 681.
- 14Asymmetric synthesis of arylkainic acids using rhodium-catalyzed carbene insertion (for details of the synthetic route, see the Supporting Information on page S2): T. Higashi, Y. Isobe, H. Ouchi, H. Suzuki, Y. Okazaki, T. Asakawa, T. Furuta, T. Wakimoto, T. Kan, Org. Lett. 2011, 13, 1089.
- 15K. Hashimoto, M. Horikawa, H. Shirahama, Tetrahedron Lett. 1990, 31, 7047.
- 16
- 16aU. Hacksell, L. E. Arvidsson, U. Svensson, J. L. G. Nilsson, D. Sanchez, H. Wikstroem, P. Lindberg, S. Hjorth, A. Carlsson, J. Med. Chem. 1981, 24, 1475;
- 16bC. Sonesson, C.-H. Lin, L. Hansson, N. Waters, K. Svensson, A. Carlsson, M. W. Smith, H. Wikstrom, J. Med. Chem. 1994, 37, 2735;
- 16cL. O. Hansson, N. Waters, S. Holm, C. Sonesson, J. Med. Chem. 1995, 38, 3121.
- 17P.-Q. Huang, X. Zheng, X.-M. Deng, Tetrahedron Lett. 2001, 42, 9039.
- 18A. Pasternak, D. Marino, P. P. Vicario, J. M. Ayala, M. A. Cascierri, W. Parsons, S. G. Mills, M. MacCoss, L. Yang, J. Med. Chem. 2006, 49, 4801.
- 19S.-B. Yang, F.-F. Gan, G.-J. Chen, P.-F. Xu, Synlett 2008, 2532.
- 20
- 20aP. Kočovský, Š. Vyskočil, I. Císařová, J. Sejbal, I. Tišlerová, M. Smrčina, G. C. Lloyd-Jones, S. C. Stephen, C. P. Butts, M. Murray, V. Langer, J. Am. Chem. Soc. 1999, 121, 7714;
- 20bJ. Yin, M. P. Rainka, X.-X. Zhang, S. L. Buchwald, J. Am. Chem. Soc. 2002, 124, 1162;
- 20cP. Dotta, P. G. A. Kumar, P. S. Pregosin, A. Albinati, S. Rizzato, Organometallics 2003, 22, 5345;
- 20dS. D. Walker, T. E. Barder, J. R. Martinelli, S. L. Buchwald, Angew. Chem. 2004, 116, 1907; Angew. Chem. Int. Ed. 2004, 43, 1871.
- 21A previous computational study reported direct CC reductive elimination from O enolates using a Josiphos ligand. No details of the calculation was given: K. Kobayashi, Y. Yamamoto, N. Miyaura, Organometallics 2011, 30, 6323.
- 22
- 22aG. R. Desiraju, Acc. Chem. Res. 1996, 29, 441;
- 22bS. J. Grabowski, J. Phys. Org. Chem. 2004, 17, 18;
- 22cT. Steiner, Angew. Chem. 2002, 114, 50;
10.1002/1521-3757(20020104)114:1<50::AID-ANGE50>3.0.CO;2-H Google ScholarAngew. Chem. Int. Ed. 2002, 41, 48;
- 22dM. C. Wahl, M. Sundaralingam, Trends Biochem. Sci. 1997, 22, 97;
- 22eR. K. Castellano, Curr. Org. Chem. 2004, 8, 845;
- 22fV. Nanda, A. Schmiedekamp, Proteins Struct. Funct. Bioinf. 2008, 70, 489;
- 22gR. Vargas, J. Garza, D. A. Dixon, B. P. Hay, J. Am. Chem. Soc. 2000, 122, 4750.
- 23Arene CH⋅⋅⋅O hydrogen bonding was implicated for controlling the E/Z ratio in Wittig reactions of triphenylphosphine ylides: R. L. Robiette, J. Richardson, V. K. Aggarwal, J. N. Harvey, J. Am. Chem. Soc. 2006, 128, 2394.
- 24
- 24aE. J. Corey, T. W. Lee, Chem. Commun. 2001, 1321;
- 24bH. Yamamoto, Tetrahedron 2007, 63, 8377;
- 24cM. N. Paddon-Row, C. D. Anderson, K. N. Houk, J. Org. Chem. 2009, 74, 861;
- 24dT. S. Thakur, M. T. Kirchner, D. Bläser, R. Boese, G. R. Desiraju, Phys. Chem. Chem. Phys. 2011, 13, 14076.
- 25
- 25aD. H. Ryu, E. J. Corey, J. Am. Chem. Soc. 2003, 125, 6388;
- 25bD. H. Ryu, E. J. Corey, J. Am. Chem. Soc. 2005, 127, 5384;
- 25cN. Y. M. Omar, N. A. Rahman, S. M. Zain, Bull. Chem. Soc. Jpn. 2011, 84, 196.
- 26J. Solà, A. Riera, X. Verdaguer, M. A. Maestro, J. Am. Chem. Soc. 2005, 127, 13629.
- 27DFT calculations revealed CH⋅⋅⋅O interactions between the phosphine methide CH bonds and the ketone carbonyl: S. L. Shi, L. W. Xu, K. Oisaki, M. Kanai, M. Shibasaki, J. Am. Chem. Soc. 2010, 132, 6638 and PhD thesis of S. L. Shi, University of Tokyo.