Structural Characterization, Infrared Spectroscopy, and Theoretical Calculations for B(C6F5)3-Stabilized Benzene–Ammonia and Benzene–Water Complexes†
The authors thank the US National Science Foundation (CHE-0948417), the National Natural Science Foundation of China (21021062), and the Fundamental Research Funds for the Central Universities of China (020514330006) for financial support, and Albemarle Corporation for donation of B(C6F5)3.
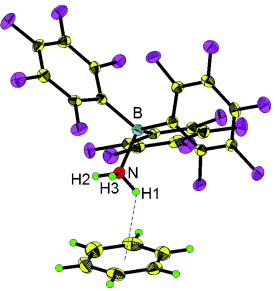
Graphical Abstract
O/NH⋅⋅⋅π hydrogen bonds: Water–benzene and ammonia–benzene complexes are stabilized by the Lewis acid B(C6F5)3 and provide rare structural (X-ray) and infrared spectroscopic data for water–benzene and ammonia–benzene complexes in the solid state (see picture). The infrared spectra of the complexes showed that the OH and NH stretching frequencies decrease significantly on benzene complexation.