Probing a Homoleptic PbS3 Coordination Environment in a Designed Peptide Using 207Pb NMR Spectroscopy: Implications for Understanding the Molecular Basis of Lead Toxicity†
Dr. Kosh P. Neupane
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA), Fax: (+1) 734-936-7628
Search for more papers by this authorProf. Dr. Vincent L. Pecoraro
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA), Fax: (+1) 734-936-7628
Search for more papers by this authorDr. Kosh P. Neupane
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA), Fax: (+1) 734-936-7628
Search for more papers by this authorProf. Dr. Vincent L. Pecoraro
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109 (USA), Fax: (+1) 734-936-7628
Search for more papers by this authorV.L.P. thanks the National Institute of Health for support of this research (R01 ES0 12236).
Graphical Abstract
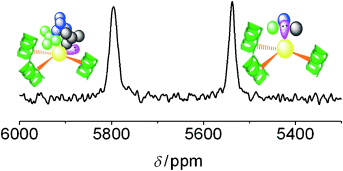
The lead-inhibited active site of a zinc-binding metalloenzyme in a thiol-rich coordination environment (PbS3) has been modeled by homoleptic three-strand coiled-coil peptides and characterized using natural-abundance 207Pb NMR spectroscopy (see picture: 207Pb NMR signals from two binding sites of the same protein). 207Pb NMR spectroscopy could thus be used to identify and characterize important human proteins associated with lead toxicity.
References
- 1B. P. Lanphear, Science 1998, 281, 1617–1618.
- 2T. J. B. Simons, Eur. J. Biochem. 1995, 234, 178–183.
- 3N. H. Zawia, T. Crumpton, M. Brydie, G. R. Reddy, M. Razmiafshari, Neurotoxicology 2000, 21, 1069–1080.
- 4T. J. B. Simons, Neurotoxicology 1993, 14, 77–85.
- 5M. Razmiafshari, N. H. Zawia, Toxicol. Appl. Pharmacol. 2000, 166, 1–12.
- 6N. H. Zawia, R. Sharan, M. Brydie, T. Oyama, T. Crumpton, Dev. Brain Res. 1999, 107, 291–298.
- 7P. T. Erskine, N. Senior, S. Awan, R. Lambert, G. Lewis, I. J. Tickle, M. Sarwar, P. Spencer, P. Thomas, M. J. Warren, P. M. Shoolingin-Jordan, S. P. Wood, J. B. Cooper, Nat. Struct. Biol. 1997, 4, 1025–1031.
- 8R. J. Andersen, R. C. diTargiani, R. D. Hancock, C. L. Stern, D. P. Goldberg, H. A. Godwin, Inorg. Chem. 2006, 45, 6574–6576.
- 9J. C. Payne, M. A. ter Horst, H. A. Godwin, J. Am. Chem. Soc. 1999, 121, 6850–6855.
- 10H. A. Godwin, Curr. Opin. Chem. Biol. 2001, 5, 223–227.
- 11J. S. Magyar, T. C. Weng, C. M. Stern, D. F. Dye, B. W. Rous, J. C. Payne, B. M. Bridgewater, A. Mijovilovich, G. Parkin, J. M. Zaleski, J. E. Penner-Hahn, H. A. Godwin, J. Am. Chem. Soc. 2005, 127, 9495–9505.
- 12B. M. Bridgewater, G. Parkin, J. Am. Chem. Soc. 2000, 122, 7140–7141.
- 13P. R. Chen, C. He, Curr. Opin. Chem. Biol. 2008, 12, 214–221.
- 14I. M. Armitage, R. T. Pajer, A. J. M. S. Uiterkamp, J. F. Chlebowski, J. E. Coleman, J. Am. Chem. Soc. 1976, 98, 5710–5712.
- 15I. M. Armitage, A. J. M. S. Uiterkamp, J. F. Chlebowski, J. E. Coleman, J. Magn. Reson. 1978, 29, 375–392.
- 16S. Forsen, C. Johansson, S. Linse, Methods Enzymol. 1993, 227, 107–118.
- 17P. D. Ellis, Science 1983, 221, 1141–1146.
- 18M. F. Summers, Coord. Chem. Rev. 1988, 86, 43–134.
- 19G. R. Dieckmann, D. K. McRorie, D. L. Tierney, L. M. Utschig, C. P. Singer, T. V. Ohalloran, J. E. PennerHahn, W. F. DeGrado, V. L. Pecoraro, J. Am. Chem. Soc. 1997, 119, 6195–6196.
- 20M. Matzapetakis, B. T. Farrer, T. C. Weng, L. Hemmingsen, J. E. Penner-Hahn, V. L. Pecoraro, J. Am. Chem. Soc. 2002, 124, 8042–8054.
- 21L. M. Utschig, J. G. Wright, G. Dieckmann, V. L. Pecoraro, T. V. O’Halloran, Inorg. Chem. 1995, 34, 2497–2498.
- 22B. Wrackmeyer, K. Horchler, Annu. Rep. NMR Spectrosc. 1990, 22, 249–306.
- 23E. S. Claudio, M. A. ter Horst, C. E. Forde, C. L. Stern, M. K. Zart, H. A. Godwin, Inorg. Chem. 2000, 39, 1391–1397.
- 24S. Rupprecht, S. J. Franklin, K. N. Raymond, Inorg. Chim. Acta 1995, 235, 185–194.
- 25J. M. Aramini, T. Hiraoki, M. Yazawa, T. Yuan, M. J. Zhang, H. J. Vogel, J. Biol. Inorg. Chem. 1996, 1, 39–48.
- 26S. Rupprecht, K. Langemann, T. Lugger, J. M. McCormick, K. N. Raymond, Inorg. Chim. Acta 1996, 243, 79–90.
- 27R. Pedrido, M. R. Bermejo, M. J. Romero, M. Vazquez, A. M. Gonzalez-Noya, M. Maneiro, M. J. Rodriguez, M. I. Fernandez, Dalton Trans. 2005, 572–579.
- 28D. L. Reger, Y. Ding, A. L. Rheingold, R. L. Ostrander, Inorg. Chem. 1994, 33, 4226–4230.
- 29E. S. Claudio, H. A. Godwin, J. S. Magyar, Prog. Inorg. Chem. 2003, 51, 1–144.
- 30J. J. I. Arsenault, P. A. W. Dean, Can. J. Chem. 1983, 61, 1516–1523.
- 31P. W. Dean, J. J. Vittal, N. C. Payne, Inorg. Chem. 1984, 23, 4232–4236.
- 32G. Christou, K. Folting, J. C. Huffman, Polyhedron 1984, 3, 1247–1253.
- 33B. T. Farrer, C. P. McClure, J. E. Penner-Hahn, V. L. Pecoraro, Inorg. Chem. 2000, 39, 5422–5423.
- 34O. Iranzo, D. Ghosh, V. L. Pecoraro, Inorg. Chem. 2006, 45, 9959–9973.
- 35M. Matzapetakis, D. Ghosh, T. C. Weng, J. E. Penner-Hahn, V. L. Pecoraro, J. Biol. Inorg. Chem. 2006, 11, 876–890.
- 36G. R. Dieckmann, D. K. McRorie, J. D. Lear, K. A. Sharp, W. F. DeGrado, V. L. Pecoraro, J. Mol. Biol. 1998, 280, 897–912.
- 37B. T. Farrer, V. L. Pecoraro, Proc. Natl. Acad. Sci. USA 2003, 100, 3760–3765.
- 38D. Ghosh, V. L. Pecoraro, Inorg. Chem. 2004, 43, 7902–7915.
- 39A. F. A. Peacock, O. Iranzo, V. L. Pecoraro, Dalton Trans. 2009, 2271–2280.
- 40M. Matzapetakis, University of Michigan (Ann Arbor), 2004.
- 41L. S. Busenlehner, N. J. Cosper, R. A. Scott, B. P. Rosen, M. D. Wong, D. P. Giedroc, Biochemistry 2001, 40, 4426–4436.
- 42L. S. Busenlehner, T.-C. Weng, J. E. Penner-Hahn, D. P. Giedroc, J. Mol. Biol. 2002, 319, 685–701.
- 43L. M. Utschig, J. W. Bryson, T. V. O’Halloran, Science 1995, 268, 380–385.
- 44J. M. Aramini, P. H. Krygsman, H. J. Vogel, Biochemistry 1994, 33, 3304–3311.
- 45A. F. A. Peacock, J. A. Stuckey, V. L. Pecoraro, Angew. Chem. 2009, 121, 7507–7510;
10.1002/ange.200902166 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7371–7374.
- 46O. Iranzo, T. Jakusch, K. H. Lee, L. Hemmingsen, V. L. Pecoraro, Chem. Eur. J. 2009, 15, 3761–3772.
- 47W. C. Chan, P. D. White, Fmoc Solid-Phase Peptide Synthesis: A Practical Approach, Oxford University Press, New York, 2000.
- 48G. L. Ellman, Arch. Biochem. Biophys. 1958, 74, 443–450.
- 49C. Cobas, J. Cruces, F. J. Sardina, MestRe-C version 2.3, Universidad de Santiago de Compostela, Spain, 2000.