An Intermolecular Palladium-Catalyzed Diamination of Unactivated Alkenes†
Dr. Álvaro Iglesias
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-224 http://www.iciq.es
Search for more papers by this authorDr. Edwin G. Pérez
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-224 http://www.iciq.es
Permanent address: Facultad de Química, Pontificia Universidad Católica de Chile, Vicuña Mackenna 4860, Casilla 306, Correo 22, Santiago (Chile)
Search for more papers by this authorProf. Dr. Kilian Muñiz
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-224 http://www.iciq.es
Search for more papers by this authorDr. Álvaro Iglesias
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-224 http://www.iciq.es
Search for more papers by this authorDr. Edwin G. Pérez
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-224 http://www.iciq.es
Permanent address: Facultad de Química, Pontificia Universidad Católica de Chile, Vicuña Mackenna 4860, Casilla 306, Correo 22, Santiago (Chile)
Search for more papers by this authorProf. Dr. Kilian Muñiz
Institute of Chemical Research of Catalonia (ICIQ), Av. Països Catalans 16, 43007 Tarragona (Spain), Fax: (+34) 977-920-224 http://www.iciq.es
Search for more papers by this authorWe thank Fundación ICIQ, the Consolider INTECAT 2010 (Project CSD2006-0003), and the Fonds der Chemischen Industrie for financial support. E.G.P. was funded by ICM Grant P05-001.
Graphical Abstract
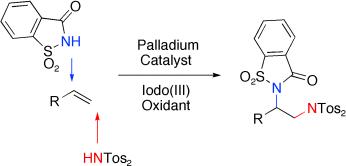
Adding 2Ns: Palladium catalysis introduces two nitrogen groups in a new regio and chemoselective diamination of non-activated alkenes that proceeds under entirely intermolecular reaction control. This palladium-catalyzed reaction employs commercial nitrogen sources in combination with a hypervalent iodo(III) reagent as oxidant (see scheme; Tos=toluenesulfonyl).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201003653_sm_miscellaneous_information.pdf2.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. Lucet, T. Le Gall, C. Mioskowski, Angew. Chem. 1998, 110, 2724;
10.1002/(SICI)1521-3757(19981002)110:19<2724::AID-ANGE2724>3.0.CO;2-4 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2580;10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 1bJ.-C. Kizirian, Chem. Rev. 2008, 108, 140;
- 1cS. R. S. S. Kotti, C. Timmons, G. Li, Chem. Biol. Drug Des. 2006, 67, 101;
- 1dK. Muñiz, New J. Chem. 2005, 29, 1371.
- 2
- 2aF. Cardona, A. Goti, Nat. Chem. 2009, 1, 269;
- 2bR. M. de Figueiredo, Angew. Chem. 2009, 121, 1212;
10.1002/ange.200804362 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 1190;
- 2cK. Muñiz, C. H. Hövelmann, J. Streuff, E. Campos-Gómez, Pure Appl. Chem. 2008, 80, 1089.
- 3First report on palladium-mediated diamination of alkenes: J.-E. Bäckvall, Tetrahedron Lett. 1975, 16, 2225.
- 4First report on the concept of metal-mediated diamination of alkenes: V. Gómez Aranda, J. Barluenga, F. Aznar, Synthesis 1974, 504.
10.1055/s-1974-23358 Google Scholar
- 5For reports on palladium-catalyzed diamination of butadienes and by allylic and homoallylic CH activation see Ref. [2a,b] and
- 5aG. L. J. Bar, G. C. Lloyd-Jones, K. I. Booker-Milburn, J. Am. Chem. Soc. 2005, 127, 7308;
- 5bH. Du, B. Zhao, Y. Shi, J. Am. Chem. Soc. 2007, 129, 762;
- 5cH. Du, W. Yuan, B. Zhao, Y. Shi, J. Am. Chem. Soc. 2007, 129, 7496;
- 5dH. Du, W. Yuan, B. Zhao, Y. Shi, J. Am. Chem. Soc. 2007, 129, 11688;
- 5eB. Wang, H. Du, Y. Shi, Angew. Chem. 2008, 120, 8348; Angew. Chem. Int. Ed. 2008, 47, 8224;
- 5fH. Du, B. Zhao, Y. Shi, J. Am. Chem. Soc. 2008, 130, 8590;
- 5gL. Hu, Y. Shi, J. Org. Chem. 2008, 73, 749; For related copper catalysis:
- 5hB. Zhao, H. Du, Y. Shi, J. Org. Chem. 2009, 74, 8392;
- 5iW. Yuan, H. Du, B. Zhao, Y. Shi, Org. Lett. 2007, 9, 2589;
- 5jB. Zhao, H. Du, Y. Shi, Org. Lett. 2008, 10, 1087;
- 5kH. Du, B. Zhao, W. Yuan, Y. Shi, Org. Lett. 2008, 10, 4231.
- 6
- 6aJ. Streuff, C. H. Hövelmann, M. Nieger, K. Muñiz, J. Am. Chem. Soc. 2005, 127, 14586;
- 6bK. Muñiz, C. H. Hövelmann, J. Streuff, J. Am. Chem. Soc. 2008, 130, 763;
- 6cK. Muñiz, J. Am. Chem. Soc. 2007, 129, 14542;
- 6dC. H. Hövelmann, J. Streuff, L. Brelot, K. Muñiz, Chem. Commun. 2008, 2334;
- 6eK. Muñiz, C. H. Hövelmann, E. Campos-Gómez, J. Barluenga, J. M. González, J. Streuff, M. Nieger, Chem. Asian J. 2008, 3, 776;
- 6fK. Muñiz, J. Streuff, P. Chávez, C. H. Hövelmann, Chem. Asian J. 2008, 3, 1248;
- 6gP. A. Sibbald, F. E. Michael, Org. Lett. 2009, 11, 1147;
- 6hP. A. Sibbald, C. F. Rosewall, R. D. Swartz, F. E. Michael, J. Am. Chem. Soc. 2009, 131, 15945.
- 7For related catalyses with nickel:
- 7aK. Muñiz, J. Streuff, C. H. Hövelmann, A. Nuñez, Angew. Chem. 2007, 119, 7255;
10.1002/ange.200702160 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7125; gold:
- 7bA. Iglesias, K. Muñiz, Chem. Eur. J. 2009, 15, 10563; copper:
- 7cY. Wen, B. Zhao, Y. Shi, Org. Lett. 2009, 11, 2365;
- 7dB. Zhao, W. Yuan, Y. Shi, Org. Lett. 2007, 9, 4943;
- 7eF. C. Sequeira, B. W. Turnpenny, S. R. Chemler, Angew. Chem. 2010, 122, 6509; Angew. Chem. Int. Ed. 2010, 49, 6356.
10.1002/ange.201003499 Google Scholar
- 8K. Muñiz, Angew. Chem. 2009, 121, 9576;
10.1002/ange.200903671 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9412.
- 9For relevant intermolecular 1,2-difunctionalization of alkenes by high-oxidation-state palladium catalysis see, for diacetoxylation:
- 9aY. Li, D. Song, V. M. Dong, J. Am. Chem. Soc. 2008, 130, 2962;
- 9bA. Wang, H. Jiang, H. Chen, J. Am. Chem. Soc. 2009, 131, 3846;
- 9cC. P. Park, J. H. Lee, K. S. Yoo, K. W. Jung, Org. Lett. 2010, 12, 2450;
- 9dfor aminofluorination: S. Qiu, T. Xu, J. Zhou, Y. Guo, G. Liu, J. Am. Chem. Soc. 2010, 132, 2856;
- 9efor aminoacetoxylation: E. J. Alexanian, C. Lee, E. J. Sorensen, J. Am. Chem. Soc. 2005, 127, 7690, and Ref. [11].
- 10Within strict terminology, the present cases can not be classed as amine-transfer reactions, as imines are employed as nitrogen sources. However, recently the term amination reactions has been used almost exclusively for this type of reaction (see, for example, Ref. [9e] and [11]). We therefore within this work refer to the term diamination for the transfer of the two nitrogen groups onto an alkene, in agreement with earlier work. Please see also Ref. [6 b] for an earlier discussion of this context.
- 11
- 11aG. Liu, S. S. Stahl, J. Am. Chem. Soc. 2006, 128, 7179;
- 11bL. Desai, M. S. Sanford, Angew. Chem. 2007, 119, 5839; Angew. Chem. Int. Ed. 2007, 46, 5737.
- 12For current reviews on iodo(III) reagents:
- 12aS. Schäfer, T. Wirth, Angew. Chem. 2010, 122, 2846;
10.1002/ange.200907134 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2786, and references therein;
- 12bV. V. Zhdankin, P. J. Stahl, Chem. Rev. 2008, 108, 5299;
- 12cM. Uyanik, K. Ishihara, Chem. Commun. 2009, 2086.
- 13Further details are included in the Supporting Information.
- 14The two imides mainly used are both economically attractive nitrogen sources: saccharin (Alfaaesar, 0.03 €/mmol) and bistosylimide (Acros, 0.32 €/mmol).
- 15D. M. Dastrup, M. P. VanBrunt, S. M. Weinreb, J. Org. Chem. 2003, 68, 4112.