Single-Crystal to Single-Crystal Cross-Linking of an Interpenetrating Chiral Metal–Organic Framework and Implications in Asymmetric Catalysis†
Dr. Liqing Ma
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA), Fax: (+1) 919-962-2388 http://www.chem.unc.edu/people/faculty/linw/wlindex.html
These authors contributed equally to this work.
Search for more papers by this authorDr. Chuan-De Wu
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA), Fax: (+1) 919-962-2388 http://www.chem.unc.edu/people/faculty/linw/wlindex.html
These authors contributed equally to this work.
Search for more papers by this authorMarcela M. Wanderley
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA), Fax: (+1) 919-962-2388 http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorProf. Wenbin Lin
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA), Fax: (+1) 919-962-2388 http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorDr. Liqing Ma
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA), Fax: (+1) 919-962-2388 http://www.chem.unc.edu/people/faculty/linw/wlindex.html
These authors contributed equally to this work.
Search for more papers by this authorDr. Chuan-De Wu
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA), Fax: (+1) 919-962-2388 http://www.chem.unc.edu/people/faculty/linw/wlindex.html
These authors contributed equally to this work.
Search for more papers by this authorMarcela M. Wanderley
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA), Fax: (+1) 919-962-2388 http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorProf. Wenbin Lin
Department of Chemistry, CB#3290, University of North Carolina, Chapel Hill, NC 27599 (USA), Fax: (+1) 919-962-2388 http://www.chem.unc.edu/people/faculty/linw/wlindex.html
Search for more papers by this authorWe thank NSF (CHE-0809776) for financial support and Kathryn deKrafft and Dr. Banu Kesanli for experimental help.
Graphical Abstract
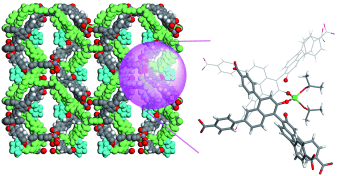
Support framework: Post-synthesis modification of an interpenetrating Zn-based chiral metal–organic framework with Ti(OiPr)4 results in a Lewis acidic catalyst (see picture; gray C, white H, red O, green Ti) with modest enantioselectivity in the asymmetric addition of diethylzinc to aldehydes. The Ti(OiPr)4-treated framework contains single-crystal to single-crystal cross-linking of the two interpenetrating networks.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201003377_sm_miscellaneous_information.pdf640.6 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aB. Moulton, M. J. Zaworotko, Chem. Rev. 2001, 101, 1629–1658;
- 1bO. R. Evans, W. Lin, Acc. Chem. Res. 2002, 35, 511–522;
- 1cS. L. James, Chem. Soc. Rev. 2003, 32, 276–288;
- 1dN. W. Ockwig, O. Delgado-Friedrichs, M. O’Keeffe, O. M. Yaghi, Acc. Chem. Res. 2005, 38, 176–182;
- 1eD. Bradshaw, J. E. Warren, M. J. Rosseinsky, Science 2007, 315, 977–980;
- 1fG. Férey, Dalton Trans. 2009, 4400–4415.
- 2
- 2aM. Dincă, J. R. Long, Angew. Chem. 2008, 120, 6870–6884;
10.1002/ange.200801163 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6766–6779;
- 2bR. Matsuda, R. Kitaura, S. Kitagawa, Y. Kubota, R. V. Belosludov, T. C. Kobayashi, H. Sakamoto, T. Chiba, M. Takata, Y. Kawazoe, Y. Mita, Nature 2005, 436, 238–241.
- 3
- 3aL. Ma, C. Abney, W. Lin, Chem. Soc. Rev. 2009, 38, 1248–1256;
- 3bJ. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen, J. T. Hupp, Chem. Soc. Rev. 2009, 38, 1450–1459.
- 4
- 4aB. L. Chen, L. B. Wang, Y. Q. Xiao, F. R. Fronczek, M. Xue, Y. J. Cui, G. D. Qian, Angew. Chem. 2009, 121, 508–511; Angew. Chem. Int. Ed. 2009, 48, 500–503;
- 4bA. J. Lan, K. H. Li, H. H. Wu, D. H. Olson, T. J. Emge, W. Ki, M. C. Hong, J. Li, Angew. Chem. 2009, 121, 2370–2374; Angew. Chem. Int. Ed. 2009, 48, 2334–2338;
- 4cZ. Xie, L. Ma, K. E. deKrafft, A. Jin, W. Lin, J. Am. Chem. Soc. 2010, 132, 922–923.
- 5
- 5aW. J. Rieter, K. M. Pott, K. M. L. Taylor, W. Lin, J. Am. Chem. Soc. 2008, 130, 11584;
- 5bK. M. L. Taylor-Pashow, J. D. Rocca, Z. Xie, S. Tran, W. Lin, J. Am. Chem. Soc. 2009, 131, 14261;
- 5cP. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. S. Chang, Y. K. Hwang, V. Marsaud, P. N. Bories, L. Cynober, S. Gil, G. Ferey, P. Couvreur, R. Gref, Nat. Mater. 2010, 9, 172–178.
- 6
- 6aC. Wu, A. Hu, L. Zhang, W. Lin, J. Am. Chem. Soc. 2005, 127, 8940–8941;
- 6bC. Wu, W. Lin, Angew. Chem. 2007, 119, 1093–1096; Angew. Chem. Int. Ed. 2007, 46, 1075–1078.
- 7L. Ma, J. M. Falkowski, C. Abney, W. Lin, Nat. Chem. 2010, DOI: .
- 8The determination of the unit cell and data collections for colorless crystals of 1 and 2 were performed on a Siemens SMART CCD diffractometer. Data for Ti-2 was collected on a Bruker SMART APEX II diffractometer. Crystallographic data for 1: Orthorhombic, space group I212121, a=13.0884(3), b=28.7131(7), c=42.8771(11) Å, V=16 113.6(7) Å3, Z=8, ρcalcd=0.858 g cm−3, μ(MoKα)=0.635 mm−1. R1(I>2σ(I))=0.0713, wR2(I>2σ(I))=0.1513, Flack parameter=0.00(3) and GOF=1.062. Crystallographic data for 2: Orthorhombic, space group I212121, a=12.7526(10), b=26.9066(19), c=44.199(3) Å, V=15 166(2) Å3, Z=8, ρcalcd=0.861 g cm−3, μ(MoKα)=0.672 mm−1. R1(I>2σ(I))=0.0492, wR2(I>2σ(I))=0.1011, Flack parameter=0.10(1) and GOF=0.890. Crystallographic data for Ti-2: Orthorhombic, space group I212121, a=13.1451(4), b=31.9683(10), c=39.8933(12) Å, V=16 344.0(9) Å3, Z=8, ρcalcd=0.805 g cm−3, μ(CuKα)=1.409 mm−1. R1(I>2σ(I))=0.0956, wR2(I>2σ(I))=0.2442, Flack parameter=0.10(6) and GOF=1.223. CCDC 772189, 772190, and 778882 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 9
- 9aV. A. Blatov, A. P. Shevchenko, V. N. Serezhkin, Russ. J. Coord. Chem. 1999, 25, 453–465;
- 9bM. O’Keeffe, N. E. Brese, Acta Cryst. A, 1992, A48, 663–669.
- 10C. Serre, F. Millange, C. Thouvenot, M. Noguès, G. Marsolier, D. Louër, G. Férey, J. Am. Chem. Soc. 2002, 124, 13519.
- 11
- 11aK. K. Tanabe, S. M. Cohen, Angew. Chem. 2009, 121, 7560–7563;
10.1002/ange.200903433 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7424–7427;
- 11bM. Banerjee, S. Das, M. Yoon, H. J. Choi, M. H. Hyun, S. M. Park, G. Seo, K. Kim, J. Am. Chem. Soc. 2009, 131, 7524–7525.
- 12
- 12aC. D. Wu, W. Lin, Angew. Chem. 2005, 117, 1994–1997; Angew. Chem. Int. Ed. 2005, 44, 1958–1961;
- 12bL. R. MacGillivray, G. S. Papaefstathiou, T. Friscić, T. D. Hamilton, D. K. Bucar, Q. Chu, D. B. Varshney, I. G. Georgiev, Acc. Chem. Res. 2008, 41, 280–291;
- 12cH. J. Park, M. P. Suh, Chem. Eur. J. 2008, 14, 8812–8821;
- 12dB. D. Chandler, G. D. Enright, K. A. Udachin, S. Pawsey, J. A. Ripmeester, D. T. Cramb, G. K. Shimizu, Nat. Mater. 2008, 7, 229–235.
- 13Related catalytic reactions have recently been reported for molecular solids, see:
- 13aA. N. Sokolov, D.-K. Bučar, J. Baltrusaitis, S. X. Gu, L. R. MacGillivray, Angew. Chem. 2010, 122, 4369–4373;
10.1002/ange.201000874 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 4273–4277;
- 13bZ. Huang, P. S. White, M. Brookhart, Nature 2010, 465, 598–601.
- 14L. Pu, H.-B. Yu, Chem. Rev. 2001, 101, 757.
- 15The Ti content (Ti/Zn=0.42) determined by the ICP-MS analysis is slightly higher than that determined from the X-ray structure (Ti/Zn=0.25), thus suggesting that there might be some disordered, unreacted Ti(OiPr)4 in the open channels of Ti-2.