Switching of a Single Boryl Center in π-Conjugated Photochromic Polyboryl Compounds and Its Impact on Fluorescence Quenching†
Dr. Chul Baik
Department of Chemistry, Queen's University, Kingston, Ontario, K7L 3N6 (Canada), Fax: (+1) 613-533-6669
Search for more papers by this authorStephen K. Murphy
Department of Chemistry, Queen's University, Kingston, Ontario, K7L 3N6 (Canada), Fax: (+1) 613-533-6669
Search for more papers by this authorProf. Dr. Suning Wang
Department of Chemistry, Queen's University, Kingston, Ontario, K7L 3N6 (Canada), Fax: (+1) 613-533-6669
Search for more papers by this authorDr. Chul Baik
Department of Chemistry, Queen's University, Kingston, Ontario, K7L 3N6 (Canada), Fax: (+1) 613-533-6669
Search for more papers by this authorStephen K. Murphy
Department of Chemistry, Queen's University, Kingston, Ontario, K7L 3N6 (Canada), Fax: (+1) 613-533-6669
Search for more papers by this authorProf. Dr. Suning Wang
Department of Chemistry, Queen's University, Kingston, Ontario, K7L 3N6 (Canada), Fax: (+1) 613-533-6669
Search for more papers by this authorThis work was supported financially by the Natural Sciences and Engineering Research Council of Canada. We thank Zachary M. Hudson for help in the preparation of this manuscript.
Graphical Abstract
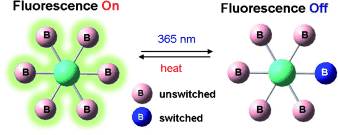
All for one and one for all: Molecules with multiple conjugated photochromic boryl units undergo photoisomerization on a single boryl unit only. This single boryl switching causes fluorescence quenching of the entire molecule, which is greatly amplified with increasing numbers of switchable boryl units in the molecule.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201003144_sm_miscellaneous_information.pdf994.4 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Wakamiya, T. Taniguchi, S. Yamaguchi, Angew. Chem. 2006, 118, 3242; Angew. Chem. Int. Ed. 2006, 45, 3170;
- 1bA. Fukazawa, H. Yamada, S. Yamaguchi, Angew. Chem. 2008, 120, 5664;
10.1002/ange.200801834 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5582;
- 1cQ. D. Liu, M. S. Mudadu, R. Thummel, Y. Tao, S. Wang, Adv. Funct. Mater. 2005, 15, 143;
- 1dY. Cui, S. Wang, J. Org. Chem. 2006, 71, 6485;
- 1eY. Cui, Q. D. Liu, D. R. Bai, W. L. Jia, Y. Tao, S. Wang, Inorg. Chem. 2005, 44, 601;
- 1fS. Wang, Coord. Chem. Rev. 2001, 215, 79;
- 1gS. F. Liu, Q. Wu, H. L. Schmider, H. Aziz, N. X. Hu, Z. Popović, S. Wang, J. Am. Chem. Soc. 2000, 122, 3671;
- 1hQ. Wu, M. Esteghamatian, N. X. Hu, Z. Popovic, G. Enright, S. Breeze, S. Wang, Angew. Chem. 1999, 111, 1039;
Angew. Chem. Int. Ed. 1999, 38, 985;
10.1002/(SICI)1521-3773(19990401)38:7<985::AID-ANIE985>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 1iQ. Wu, M. Esteghamatian, N. X. Hu, Z. Popovic, G. Enright, S. Wang, Y. Tao, M. D’Iorio, Chem. Mater. 2000, 12, 79;
- 1jH. Y. Chen, Y. Chi, C. S. Liu, J. K. Yu, Y. M. Cheng, K. S. Chen, P. T. Chou, S. M. Peng, G. H. Lee, A. J. Carty, S. J. Yeh, C. T. Chen, Adv. Funct. Mater. 2005, 15, 567;
- 1kH. Y. Zhang, C. Huo, K. Q. Ye, P. Zhang, W. J. Tian, Y. Wang, Inorg. Chem. 2006, 45, 2788;
- 1lZ. L. Zhang, H. Bi, Y. Zhang, D. D. Yao, H. Z. Gao, Y. Fan, H. Y. Zhang, Y. Wang, Y. P. Wang, Z. Y. Chen, D. G. Ma, Inorg. Chem. 2009, 48, 7230;
- 1mH. Y. Zhang, C. Huo, J. Y. Zhang, P. Zhang, W. J. Tian, Y. Wang, Chem. Commun. 2006, 281.
- 2
- 2aY. Qin, I. Kiburu, S. Shah, F. Jäkle, Macromolecules 2006, 39, 9041;
- 2bY. Qin, I. Kiburu, S. Shah, F. Jaekle, Org. Lett. 2006, 8, 5227;
- 2cY. Qin, C. Pagba, P. Piotrowiak, F. Jäkle, J. Am. Chem. Soc. 2004, 126, 7015;
- 2dT. Iijima, T. Yamamoto, Macromol. Rapid Commun. 2004, 25, 669;
- 2eA. Meyers, M. Weck, Macromolecules 2003, 36, 1766;
- 2fT. Yamamoto, I. Yamaguchi, Polym. Bull. 2003, 50, 55.
- 3
- 3aM. Elbing, G. C. Bazan, Angew. Chem. 2008, 120, 846;
10.1002/ange.200703722 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 834;
- 3bT. W. Hudnall, C.-W. Chiu, F. P. Gabbaï, Acc. Chem. Res. 2009, 42, 388;
- 3cC. D. Entwistle, T. B. Marder, Chem. Mater. 2004, 16, 4574;
- 3dG. J. Zhou, C. L. Ho, W. Y. Wong, Q. Wang, D. G. Ma, L. X. Wang, Z. Y. Lin, T. B. Marder, A. Beeby, Adv. Funct. Mater. 2008, 18, 499;
- 3eF. Jäkle, Coord. Chem. Rev. 2006, 250, 1107;
- 3fZ. M. Hudson, S. Wang, Acc. Chem. Res. 2009, 42, 1584;
- 3gY. Shirota, J. Mater. Chem. 2000, 10, 1.
- 4
- 4aY. L. Rao, H. Amarne, S. B. Zhao, T. M. McCormick, S. Martić, Y. Sun, R. Y. Wang, S. Wang, J. Am. Chem. Soc. 2008, 130, 12898;
- 4bC. Baik, Z. M. Hudson, H. Amarne, S. Wang, J. Am. Chem. Soc. 2009, 131, 14549;
- 4cH. Amarne, C. Baik, S. K. Murphy, Chem. Eur. J. 2010, 16, 4750.
- 5A. S. Hay, J. Org. Chem. 1962, 27, 3320.
- 6C. Grave, D. Lentz, A. Schafer, P. Samor, J. P. Rabe, P. Franke, A. D. Schluter, J. Am. Chem. Soc. 2003, 125, 6907.
- 7CCDC 777277 (B2), 777278 (B2′), and 777279 (B6) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 8M. Kawa, Top. Curr. Chem. 2003, 228, 193, and references therein.
- 9M. Kasha, Discuss. Faraday Soc. 1950, 9, 14.
- 10J. Areephong, H. Logtenberg, W. R. Browne, B. L. Feringa, Org. Lett. 2010, 12, 2132.
- 11B. T. Holmes, P. Deb, W. T. Pennington, T. W. Hanks, J. Polym. Res. 2006, 13, 133.
- 12N. J. Demas, G. A. Crosby, J. Am. Chem. Soc. 1970, 92, 7262.
- 13M. J. Frisch et al. Gaussian 03, revision C.02; Gaussian, Inc.: Wallingford, CT, 2004.