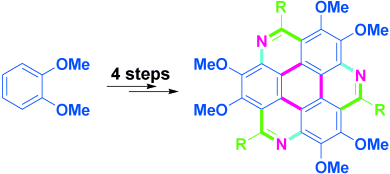
1,5,9-Triazacoronenes: A Family of Polycyclic Heteroarenes Synthesized by a Threefold Pictet–Spengler Reaction†
Correction(s) for this article
-
Corrigendum: 1,5,9-Triazacoronenes: A Family of Polycyclic Heteroarenes Synthesized by a Threefold Pictet–Spengler Reaction
- Volume 49Issue 51Angewandte Chemie International Edition
- pages: 9803-9803
- First Published online: December 14, 2010
Prof. Dr. Junfa Wei
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorBo Han
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorQiang Guo
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorDr. Xianying Shi
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorProf. Wenliang Wang
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorNi Wei
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorProf. Dr. Junfa Wei
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorBo Han
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorQiang Guo
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorDr. Xianying Shi
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorProf. Wenliang Wang
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorNi Wei
School of Chemistry and Materials Science, Key Laboratory for Macromolecular Science of Shaanxi Province, Shaanxi Normal University, Xi'an, 710062 (P.R. China), Fax: (+86) 29-8530-7774
Search for more papers by this authorThis work was financially supported by the National Foundation of Natural Science in China (Grant No. 21072123, 20873079, and 20906059).
Graphical Abstract
We are family: Triazacoronene derivatives have been synthesized in four steps from veratrole by using a threefold Pictet–Spengler reaction as the key step (see picture). They have good photophysical and electronical properties, thermal stability, and solubility, thus rendering them promising candidates as electron-transport materials.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201002369_sm_miscellaneous_information.pdf2.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews, see:
- 1aS. Allard, M. Forster, B. Souharce, H. Thiem, U. Scherf, Angew. Chem. 2008, 120, 4138–4167;
10.1002/ange.200701920 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4070–4098;
- 1bS. Günes, H. Neugebauer, N. S. Sariciftci, Chem. Rev. 2007, 107, 1324–1338;
- 1cW. Pisula, A. K. Mishra, J. Li, M. Baumgarten, K. Müllen, Org. Photovoltaics 2008, 93–128;
- 1dB. C. Thompson, J. M. J. Frechet, Angew. Chem. 2008, 120, 62–82;
10.1002/ange.200702506 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 58–77;
- 1eJ. E. Anthony, Angew. Chem. 2008, 120, 460–492;
10.1002/ange.200604045 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 452–483;
- 1fY. Shirota, H. Kageyama, Chem. Rev. 2007, 107, 953–1010;
- 1gA. C. Arias, J. D. MacKenzie, I. McCulloch, J. Rivnay, A. Salleo, Chem. Rev. 2010, 110, 3–24;
- 1hX. Meng, W. Zhu, H. Tian, Opt. Sci. Eng. 2008, 133, 129–172;
- 1iA. C. Grimsdale, K. L. Chan, R. E. Martin, P. G. Jokisz, A. B. Holmes, Chem. Rev. 2009, 109, 897–1091;
- 1jM. Mas-Torrent, C. Rovira, Chem. Soc. Rev. 2008, 37, 827–838;
- 1kA. P. Kulkarni, C. J. Tonzola, A. Babel, S. A. Jenekhe, Chem. Mater. 2004, 16, 4556–4573.
- 2For examples, see:
- 2aT. Yamamoto, T. Fukushima, A. Kosaka, W. Jin, Y. Yamamoto, N. Ishii, T. Aida, Angew. Chem. 2008, 120, 1696–1699; Angew. Chem. Int. Ed. 2008, 47, 1672–1675;
- 2bY. Fogel, M. Kastler, Z. Wang, D. Andrienko, G. J. Bodwell, K. Müllen, J. Am. Chem. Soc. 2007, 129, 11743–11749;
- 2cJ.-L. Wang, Y. Zhou, Y. Li, J. Pei, J. Org. Chem. 2009, 74, 7449–7456;
- 2dJ.-L. Wang, J. Yan, Z.-M. Tang, Q. Xiao, Y. Ma, J. Pei, J. Am. Chem. Soc. 2008, 130, 9952–9962;
- 2eB. Ma, C. H. Woo, Y. Miyamoto, J. M. J. Frechet, Chem. Mater. 2009, 21, 1413–1417.
- 3
- 3aM. Takase, V. Enkelmann, D. Sebastiani, M. Baumgarten, K. Müllen, Angew. Chem. 2007, 119, 5620–5623;
10.1002/ange.200701452 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5524–5527;
- 3bD. Wu, W. Pisula, M. C. Haberecht, X. Feng, K. Müllen, Org. Lett. 2009, 11, 5686–5689;
- 3cS. Barlow, Q. Zhang, B. R. Kafarani, C. Risko, F. Amy, C. K. Chan, B. Domercq, Z. A. Starikova, M. Y. Antipin, T. V. Timofeeva, B. Kippelen, J. L. Bredas, A. Kahn, S. R. Marder, Chem. Eur. J. 2007, 13, 3537–3547;
- 3dM. J. D. Bosdet, W. E. Piers, T. S. Sorensen, M. Parvez, Angew. Chem. 2007, 119, 5028–5031;
10.1002/ange.200700591 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4940–4943;
- 3eS. Alibert-Fouet, I. Seguy, J. F. Bobo, P. Destruel, H. Bock, Chem. Eur. J. 2007, 13, 1746–1753;
- 3fW. Jiang, Y. Li, W. Yue, Y. Zhen, J. Qu, Z. Wang, Org. Lett. 2010, 12, 228–231;
- 3gY. Sun, L. Tan, S. Jiang, H. Qian, Z. Wang, J. Am. Chem. Soc. 2007, 129, 1882–1883;
- 3hJ.-F. Wei, X.-W. Jia, J. Yu, X.-Y Shi, C.-J. Zhang, Z.-G. Chen, Chem. Commun. 2009, 4714–4716.
- 4A. Léger, L. Hendecourt in Polycyclic Aromatic Hydrocarbons and Astrophysics (Eds.: ), Reidel, Dordrecht, The Netherlands, 1987, p. 223.
10.1007/978-94-009-4776-4_21 Google Scholar
- 5aA. K. Geim, K. S. Novoselov, Nat. Mater. 2007, 6, 183–191;
- 5bK. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva, A. A. Firsov, Science 2004, 306, 666–669;
- 5cJ. M. Schulman, R. L. Disch, J. Phys. Chem. A 1997, 101, 9176–9179;
- 5dM. D. Watson, M. G. Debije, J. M. Warman, K. Müllen, J. Am. Chem. Soc. 2004, 126, 766–771;
- 5eB. K. Spraul, S. Suresh, S. Glaser, D. Perahia, J. Ballato, D. W. Smith, Jr., J. Am. Chem. Soc. 2004, 126, 12772–12773;
- 5fV. Palermo, S. Morelli, C. Simpson, K. Müllen, P. Samori, J. Mater. Chem. 2006, 16, 266–271;
- 5gH. C. Shen, J. M. Tang, H. K. Chang, C. W. Yang, R. S. Liu, J. Org. Chem. 2005, 70, 10113–10116;
- 5hW. W. H. Wong, D. J. Jones, C. Yan, S. E. Watkins, S. King, S. A. Haque, X. Wen, K. P. Ghiggino, A. B. Holmes, Org. Lett. 2009, 11, 975–978.
- 6
- 6aR. Rieger, M. Kastler, V. Enkelmann, K. Müllen, Chem. Eur. J. 2008, 14, 6322–6325;
- 6bS. Xiao, J. Tang, T. Beetz, X. Guo, N. Tremblay, T. Siegrist, Y. Zhu, M. Steigerwald, C. Nuckolls, J. Am. Chem. Soc. 2006, 128, 10700–10701;
- 6cA. M. van de Craats, J. M. Warman, A. Fechtenkötter, J. D. Brand, M. A. Harbison, K. Müllen, Adv. Mater. 1999, 11, 1469–1472;
10.1002/(SICI)1521-4095(199912)11:17<1469::AID-ADMA1469>3.0.CO;2-K CAS Web of Science® Google Scholar
- 6dH.-P. Jia, S.-X. Liu, L. Sanguinet, E. Levillain, S. Decurtins, J. Org. Chem. 2009, 74, 5727–5729;
- 6eJ. L. Mynar, T. Yamamoto, A. Kosaka, T. Fukushima, N. Ishii, T. Aida, J. Am. Chem. Soc. 2008, 130, 1530–1531.
- 7
- 7aS. Tokita, K. Hiruta, K. Kitahara, H. Nishi, Synth. Comm. 1982, 229–231;
- 7bS. Tokita, K. Hiruta, K. Kitahara, H. Nishi, Bull. Chem. Soc. Jpn. 1982, 55, 3933–3934.
- 8For Pictet–Spengler condensations, see:
- 8aE. D. Cox, J. M. Cook, Chem. Rev. 1995, 95, 1797–1842;
- 8bJ. Royer, M. Bonin, L. Micouin, Chem. Rev. 2004, 104, 2311–2352.
- 9G. R. Newkome, C. D. Shreiner, Polymer 2008, 49, 1–173.
- 10N. Boden, R. C. Borner, R. J. Bushby, A. N. Cammidge, M. V. Jesudason, Liq. Cryst. 1993, 15, 851–858.
- 11CCDC 788011 (4 b) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. Crystal data for 4 b: single crystals suitable for X-ray diffraction were obtained by slowly evaporating a solution of 4 b in tetrahydrofuran over one week. C48H39N3O9⋅2.5(C4H8O), M=982.08, Triclinic, space group
 , a=11.993(4), b=13.599(4) Å, c=18.240(6) Å, α=111.631(4)°, β=107.684(4)°, γ=90.120(4)°, V=2612.8(14) Å3, T=296(2) K, Z=2, μ(MoKα)=0.71073 Å, colorless square-plates, crystal dimensions 0.40×0.25×0.19 mm, crystal density 1.248 Mg m−3. Full matrix least-squares based on F2, gave R1=0.0779 and ωR2=0.2410 for 9092 (I≥2σ(I)), GOF=1.033 for 686 parameters.
, a=11.993(4), b=13.599(4) Å, c=18.240(6) Å, α=111.631(4)°, β=107.684(4)°, γ=90.120(4)°, V=2612.8(14) Å3, T=296(2) K, Z=2, μ(MoKα)=0.71073 Å, colorless square-plates, crystal dimensions 0.40×0.25×0.19 mm, crystal density 1.248 Mg m−3. Full matrix least-squares based on F2, gave R1=0.0779 and ωR2=0.2410 for 9092 (I≥2σ(I)), GOF=1.033 for 686 parameters.
- 12
- 12aM. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson, P. E. Forrest, Appl. Phys. Lett. 1999, 75, 4–6;
- 12bH. Xin, F. Y. Li, M. Shi, Z. Q. Bian, C. H. Huang, J. Am. Chem. Soc. 2003, 125, 7166–7167.
- 13
- 13aH.-B. Bürgi, K. K. Baldridge, K. Hardcastle, N. L. Frank, P. Gantzel, J. S. Siegel, J. Ziller, Angew. Chem. 1995, 107, 1575–1577; Angew. Chem. Int. Ed. Engl. 1995, 34, 1454–1456;
- 13bC. R. Newman, C. D. Frisbie, D. A. da Silva Filho, J. L. Bredas, P. C. Ewbank, K. R. Mann, Chem. Mater. 2004, 16, 4436–4451.
- 14
- 14aJ. I. Kim, I. S. Shin, H. Kim, J. K. Lee, J. Am. Chem. Soc. 2005, 127, 1614–1615;
- 14bX. Liu, L. Shi, W. Niu, H. Li, G. Xu, Angew. Chem. 2007, 119, 425–428; Angew. Chem. Int. Ed. 2007, 46, 421–424;
- 14cC. Booker, X. Wang, S. Haroun, J. Zhou, M. Jennings, B. L. Pagenkopf, Z. Ding, Angew. Chem. 2008, 120, 7845–7849;
10.1002/ange.200802034 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7731–7735;
- 14dK. M. Omer, S.-Y. Ku, K.-T. Wong, A. J. Bard, J. Am. Chem. Soc. 2009, 131, 10733–10741.
- 15
- 15aC.-G. Zhan, J. A. Nichols, D. A. Dixon, J. Phys. Chem. A 2003, 107, 4184–4195;
- 15bV. Lukeš, R. Šolc, H. Lischka, H. F. Kauffmann, J. Phys. Chem. A 2009, 113, 14141–14149;
- 15cC. Pérez-Bolívar, V. A. Montes, P. Anzenbacher, Jr., Inorg. Chem. 2006, 45, 9610–9612.
- 16L. W. Convert, H. Adkins, J. Am. Chem. Soc. 1932, 54, 4116–4117.
10.1021/ja01349a510 Google Scholar