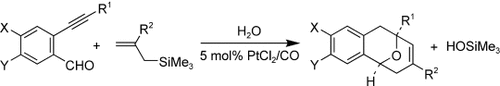
PtII-Catalyzed Synthesis of 9-Oxabicyclo[3.3.1]nona-2,6-dienes from 2-Alkynyl-1-carbonylbenzenes and Allylsilanes by an Allylation/Annulation Cascade†
Sabyasachi Bhunia
Department of Chemistry, National Tsing Hua University, Hsinchu 30043 (Taiwan), Fax: (+886) 3571-1082
Search for more papers by this authorKuo-Chang Wang
Department of Chemistry, National Tsing Hua University, Hsinchu 30043 (Taiwan), Fax: (+886) 3571-1082
Search for more papers by this authorRai-Shung Liu Prof. Dr.
Department of Chemistry, National Tsing Hua University, Hsinchu 30043 (Taiwan), Fax: (+886) 3571-1082
Search for more papers by this authorSabyasachi Bhunia
Department of Chemistry, National Tsing Hua University, Hsinchu 30043 (Taiwan), Fax: (+886) 3571-1082
Search for more papers by this authorKuo-Chang Wang
Department of Chemistry, National Tsing Hua University, Hsinchu 30043 (Taiwan), Fax: (+886) 3571-1082
Search for more papers by this authorRai-Shung Liu Prof. Dr.
Department of Chemistry, National Tsing Hua University, Hsinchu 30043 (Taiwan), Fax: (+886) 3571-1082
Search for more papers by this authorWe thank the National Science Council, Taiwan for support of this work.
Graphical Abstract
Platinum is key: A new catalytic synthesis of 9-oxabicyclo[3.3.1]nona-2,6-dienes from readily available 2-alkynyl-1-carbonylbenzenes, allylsilanes, and water is reported (see scheme). This reaction sequence is proposed to proceed through a series of three reactions, including allylation of the carbonyl group, hydroalkoxylation of the alkyne, and a new ene-oxonium annulation.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200800826_sm_miscellaneous_information.pdf6.4 MB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews and leading references, see
- 1aH. J. Knölker, N. Foitzik, C. Gabler, R. Graf, Synthesis 1999, 145–151;
- 1bH. J. Knolker, J. Prakt. Chem. 1997, 339, 304;
- 1cR. L. Danheiser, B. R. Dixon, R. W. Gleason, J. Org. Chem. 1992, 57, 6094;
- 1dJ. S. Panek, M. Yang, J. Am. Chem. Soc. 1991, 113, 9868.
- 2Selected examples, see
- 2aJ. M. Tinsley, W. R. Roush, J. Am. Chem. Soc. 2005, 127, 10818;
- 2bS. R. Angle, N. A. El-Said, J. Am. Chem. Soc. 2002, 124, 3608.
- 3S. R. Angle, N. A. El-Said, J. Am. Chem. Soc. 1999, 121, 10211.
- 4For addition of allylsilanes or allylstannanes to carbonyl derivatives by using AgI, CuII, PtII, and AuI, see selected examples:
- 4aA. Yanagisawa, H. Nakashima, A. Ishiba, H. Yamamoto, J. Am. Chem. Soc. 1996, 118, 4723;
- 4bM. Wadamoto, N. Ozasa, A. Yanagisawa, H. Yamamoto, J. Org. Chem. 2003, 68, 5593;
- 4cA. Yanagisawa, H. Kageyama, Y. Nakatsuka, K. Asakawa, Y. Matsumoto, H. Yamamoto, Angew. Chem. 1999, 111, 3916;
10.1002/(SICI)1521-3757(19991216)111:24<3916::AID-ANGE3916>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3701;10.1002/(SICI)1521-3773(19991216)38:24<3701::AID-ANIE3701>3.0.CO;2-D CAS PubMed Web of Science® Google Scholar
- 4dJ. Kruger, E. M. Carreira, J. Am. Chem. Soc. 1998, 120, 837;
- 4eS. Obika, H. Kono, Y. Yasui, R. Yanada, Y. Takemoto, J. Org. Chem. 2007, 72, 4462.
- 5C.-C. Lin, T.-M. Deng, A. Odedra, R.-S. Liu, J. Am. Chem. Soc. 2007, 129, 3798.
- 6
- 6aB. Wünsch, M. Zott, G. Höfner, Arch. Pharm. 1992, 325, 733;
- 6bB. Wünsch, M. Zott, G. Höfner, Arch. Pharm. 1993, 326, 823;
- 6cC. Ketterer, S. Grimme, E. Weckert, B. Wünsch, Tetrahedron: Asymmetry 2006, 17, 3046.
- 7
- 7aC. Maurin, F. Bailly, P. Cortelle, Curr. Med. Chem. 2003, 10, 1795;
- 7bR. Dupont, L. Jeanson, J.-F. Mouscadet, P. Cotelle, Bioorg. Med. Chem. Lett. 2001, 11, 3175;
- 7cR. Dupont, P. Cotelle, Tetrahedron Lett. 1998, 39, 8457.
- 8In wet dichloroethane (25 °C, 12 h), AgOTf, PPh3AuSbF6, and [PPh3AuOTf] led to complete consumption of starting aldehyde 1 a, but only [PPh3AuOTf] gave desired oxatricyclic species 3 in a 25 % yield.
- 9For the PtCl2/CO catalysis, see
- 9aA. Fürstner, C. Aïssa, J. Am. Chem. Soc. 2006, 128, 6306;
- 9bA. Fürstner, P. W. Davies, J. Am. Chem. Soc. 2005, 127, 15024;
- 9cB. P. Taduri, S. M. A. Sohel, H.-M. Cheng, G.-Y. Lin, R.-S. Liu, Chem. Commun. 2007, 2530–2532;
- 9dH.-K. Chang, S. Datta, A. Das, R.-S. Liu, Angew. Chem. 2007, 119, 4828; Angew. Chem. Int. Ed. 2007, 46, 4744.
- 10The x-ray crystallographic data for compound 3 c has been deposited at the Cambridge Crystallographic Data Center (CCDC-677837 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif), and 1H NOE spectra of key compounds are provided in the Supporting Information.
- 111,2-dihydronaphthalene 6 was formed from [4+2] cycloaddition of benzopyrinium C and 1-methylstyrene (see below); this olefin arose from decomposition of allylsilane with a Brønsted acid when water was present.[13] In a control experiment, we obtained compound 6 (86 % yield) from PtCl2/CO-catalyzed cyclization of 4 c with 1-methylstyrene in hot toluene. See the leading paper: N. Asao, T. Kasahara, Y. Yamamoto, Angew. Chem. 2003, 115, 3628;
10.1002/ange.200351390 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 3504.
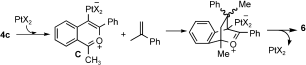
- 12For the mechanism of formation of chrysene 7, see A. Das, H.-H. Liao R.-S. Liu, J. Org. Chem. 2007, 72, 9214.
- 13For similar observations, see
- 13aY.-G. Hsu, S. Datta, C.-M. Ting, R.-S. Liu, Org. Lett. 2008, 10, 521;
- 13bS. Porcel, V. Löpez-Carrilo, C. Garcia-Yebra, A. M. Echavarren, Angew. Chem. 2008, 120, 1909;
10.1002/ange.200704500 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1883.
- 14For formation of 1H-isochromenes from hydroalkoxylation of alkyne, see reference [5e] and selected examples:
- 14aX. Yao, C.-J. Li, Org. Lett. 2006, 8, 1953;
- 14bN. Asao, T. Nogami, K. Takahashi, Y. Yamamoto, J. Am. Chem. Soc. 2002, 124, 764;
- 14cN. Asao, C. S. Chan, K. Takahashi, Y. Yamamoto, Tetrahedron 2005, 61, 11322.
- 15The calculations were done with Gaussian-98 package at level B3LYP/6-31+G*; details are provided in the Supporting information.
- 16For metal-catalyzed addition of a tethered phenol or enol to an enol ether of 1H-isochromenes, see: A. Beeler, S. Su, C. A. Singleton, J. A. Proco, Jr., J. Am. Chem. Soc. 2007, 129, 1413.
- 17Ketones are generally inactive toward metal-catalyzed allylation reactions.[1] The high yields of oxatricyclic products 5 a–5 d may arise from addition of allylsilane to benzopyrilium species C,[11] rather than by the mechanism proposed in Scheme 2. We thank one reviewer for this valuable comment.