Enantioselective Total Synthesis of the Melodinus Alkaloid (+)-Meloscine†
Philipp Selig
Department of Chemistry, Technische Universität München, Lichtenbergstrasse 4, 85747 Garching (Germany), Fax: (+49) 89-2891-3315
Search for more papers by this authorThorsten Bach Prof. Dr.
Department of Chemistry, Technische Universität München, Lichtenbergstrasse 4, 85747 Garching (Germany), Fax: (+49) 89-2891-3315
Search for more papers by this authorPhilipp Selig
Department of Chemistry, Technische Universität München, Lichtenbergstrasse 4, 85747 Garching (Germany), Fax: (+49) 89-2891-3315
Search for more papers by this authorThorsten Bach Prof. Dr.
Department of Chemistry, Technische Universität München, Lichtenbergstrasse 4, 85747 Garching (Germany), Fax: (+49) 89-2891-3315
Search for more papers by this authorThis project was supported by the Deutsche Forschungsgemeinschaft as part of the Schwerpunktprogramm Organokatalyse (Ba 1372-10), by the Studienstiftung des Deutschen Volkes (doctoral fellowship to P.S.), and by the Fonds der Chemischen Industrie.
Graphical Abstract
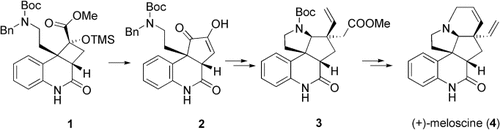
Enantioselective synthesis in a new light: The template-controlled [2+2] photocycloaddition leading to product 1 is the first example of this type of reaction in natural product synthesis. In addition, a retro-benzilic acid rearrangement (→2), a Claisen rearrangement (→3), and a ring-closing metathesis played decisive roles in the synthesis of the alkaloid (+)-meloscine (4).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200800693_sm_miscellaneous_information.pdf461.3 KB | miscellaneous information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Hesse, Alkaloide, Verlag Helvetica Chimica Acta, Zürich, 2000.
- 2
- 2aK. Bernauer, G. Englert, W. Vetter, Experientia 1965, 21, 374–375;
- 2bM. Plat, M. Hachem-Mehri, M. Koch, U. Scheidegger, P. Potier, Tetrahedron Lett. 1970, 11, 3395–3398;
10.1016/S0040-4039(01)98486-2 Google Scholar
- 2cM. Daudon, M. H. Mehri, M. M. Plat, E. W. Hagaman, E. Wenkert, J. Org. Chem. 1976, 41, 3275–3278;
- 2dH. Mehri, A. O. Diallo, M. Plat, Phytochemistry 1995, 40, 1005–1006.
- 3L.-W. Gou, Y.-L. Zhou, Phytochemistry 1993, 34, 563–566.
- 4
- 4aG. Hugel, J. Lévy, J. Org. Chem. 1984, 49, 3275–3277;
- 4bG. Palmisano, B. Danieli, G. Lesma, R. Riva, S. Riva, F. Demartin, N. Masciocchi, J. Org. Chem. 1984, 49, 4138–4143;
- 4cG. Hugel, J. Lévy, J. Org. Chem. 1986, 51, 1594–1595.
- 5K. Bernauer, G. Englert, W. Vetter, E. Weiss, Helv. Chim. Acta 1969, 52, 1886–1905.
- 6W. E. Oberhänsli, Helv. Chim. Acta 1969, 52, 1905–1911.
- 7
- 7aL. E. Overman, G. M. Robertson, A. J. Robichaud, J. Org. Chem. 1989, 54, 1236–1238;
- 7bL. E. Overman, G. M. Robertson, A. J. Robichaud, J. Am. Chem. Soc. 1991, 113, 2598–2610.
- 8Synthetic studies:
- 8aA. G. Schultz, M. Dai, Tetrahedron Lett. 1999, 40, 645–648;
- 8bS. E. Denmark, J. J. Cottell, Adv. Synth. Catal. 2006, 348, 2397–2402.
- 9For recent applications of photocycloaddition reactions in natural product synthesis, see: J. Iriondo-Alberdi, M. F. Greaney, Eur. J. Org. Chem. 2007, 4801–4815.
- 10 Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2005.
- 11
- 11aS. Brandes, P. Selig, T. Bach, Synlett 2004, 2588–2590;
- 11bP. Selig, T. Bach, J. Org. Chem. 2006, 71, 5662–5673.
- 12
- 12aT. Bach, H. Bergmann, K. Harms, Angew. Chem. 2000, 112, 2391–2393;
Angew. Chem. Int. Ed. 2000, 39, 2302–2304;
10.1002/1521-3773(20000703)39:13<2302::AID-ANIE2302>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 12bT. Bach, H. Bergmann, J. Am. Chem. Soc. 2000, 122, 11525–11526;
- 12cT. Bach, H. Bergmann, B. Grosch, K. Harms, E. Herdtweck, Synthesis 2001, 1395–1405.
- 13P. J. Connolly, C. H. Heathcock, J. Org. Chem. 1985, 50, 4135–4144.
- 14
- 14aT. Hatsui, S.-Y. Ikeda, H. Takeshita, Chem. Lett. 1992, 1891–1894;
- 14bT. Hatsui, J.-J. Wang, H. Takeshita, Bull. Chem. Soc. Jpn. 1994, 67, 2507–2513.
- 15X. Creary, P. A. Inocencio, T. L. Underiner, R. Kostromin, J. Org. Chem. 1985, 50, 1932–1938.
- 16L. F. Tietze, G. Brasche, K. Gericke, Domino Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2006.
10.1002/9783527609925 Google Scholar
- 17
- 17aC. Hartmann, V. Meyer, Ber. Dtsch. Chem. Ges. 1893, 26, 1727–1732;
10.1002/cber.189302602109 Google Scholar
- 17bM. Frigerio, M. Santagostino, S. Sputore, J. Org. Chem. 1999, 64, 4537–4538;
- 17cJ. D. More, N. S. Finney, Org. Lett. 2002, 4, 3001–3003.
- 18
- 18aD. P. Curran, M. J. Totleben, J. Am. Chem. Soc. 1992, 114, 6050–6058;
- 18bM. Nishida, H. Hayashi, Y. Yamaura, E. Yanaginuma, O. Yonemitsu, A. Nishida, N. Kawahara Tetrahedron Lett. 1995, 36, 269–272;
- 18cJ. Cossy, L. Tresnard, D. G. Pardo, Eur. J. Org. Chem. 1999, 1925–1933.
10.1002/(SICI)1099-0690(199908)1999:8<1925::AID-EJOC1925>3.0.CO;2-# CAS Web of Science® Google Scholar
- 19
- 19aY. Yamamoto, K. Maruyama, J. Am. Chem. Soc. 1978, 100, 3240–3241;
- 19bG. L. Leotta III, L. E. Overman, G. S. Welmaker, J. Org. Chem. 1994, 59, 1946.
- 20 The Claisen Rearrangement: Methods and Applications (Eds.: ), Wiley-VCH, Weinheim, 2007.
- 21W. S. Johnson, L. Werthemann, W. R. Bartlett, T. J. Brocksom, T.-T. Li, D. J. Faulkner, M. R. Petersen, J. Am. Chem. Soc. 1970, 92, 741–743.
- 22For applications of ring-closing metathesis in total synthesis, see:
- 22aA. Gradillas, J. Pérez-Castells; Angew. Chem. 2006, 118, 6232–6247; Angew. Chem. Int. Ed. 2006, 45, 6086–6101; Angew. Chem. 2006, 118, 6232–6247; Angew. Chem. Int. Ed. 2006, 45, 6086–6101;
- 22bK. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. 2005, 117, 4564–4601;
10.1002/ange.200500369 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 4490–4527;
- 22cA. Deiters, S. F. Martin, Chem. Rev. 2004, 104, 2199–2238.
- 23M. Scholl, T. M. Trnka, J. P. Morgan, R. H. Grubbs, Tetrahedron Lett. 1999, 40, 2247–2250.
- 24K. B. Sharpless, M. W. Young, J. Org. Chem. 1975, 40, 947–949.
- 25M. Daudon, M. H. Meri, M. M. Plat, E. W. Hagaman, F. M. Schell, E. Wenkert, J. Org. Chem. 1975, 40, 2838–2839.