Self-Assembly of a Highly Stable, Topologically Interesting Metallamacrocycle by Bridging Gold(I) Ions with Pyridyl-2,6-diphenyl2− and Diphosphanes†
Steven C. F. Kui Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorJie-Sheng Huang Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorRaymond Wai-Yin Sun Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorNianyong Zhu Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorChi-Ming Che Prof. Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorSteven C. F. Kui Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorJie-Sheng Huang Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorRaymond Wai-Yin Sun Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorNianyong Zhu Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorChi-Ming Che Prof. Dr.
Department of Chemistry and Open Laboratory of Chemical Biology, Institute of Molecular Technology for Drug Discovery and Synthesis, The University of Hong Kong, Pokfulam Road, Hong Kong, P.R. China, Fax: (+852) 2857-1586
Search for more papers by this authorThis study was supported by The University of Hong Kong (University Development Fund), the Hong Kong Research Grants Council, and the University Grants Committee of the Hong Kong SAR of China (Area of Excellence Scheme, AoE/P-10/01).
Graphical Abstract
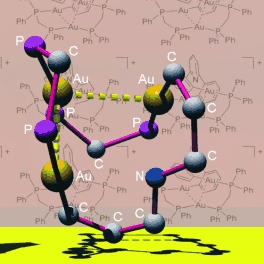
A Möbius arrangement of the bonds directly attached to a metallamacrocycle was observed for the AuI complex [Au3(CNC)(μ-Ph2PCH2PPh2)2]+, which was self-assembled by treating the lithium salt of pyridyl-2,6-diphenyl2− (CNC) with [Au2Cl2(μ-Ph2PCH2PPh2)]. The trinuclear AuI complex has intramolecular Au⋅⋅⋅Au and CH⋅⋅⋅π interactions, exhibits a remarkable stability in solution, and is cytotoxic toward cancer cell lines.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z600794_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. B. Amabilino, J. F. Stoddart, Chem. Rev. 1995, 95, 2725;
- 1bC. Piguet, G. Bernardinelli, G. Hopfgartner, Chem. Rev. 1997, 97, 2005;
- 1cF. M. Raymo, J. F. Stoddart, Chem. Rev. 1999, 99, 1643;
- 1dS. Leininger, B. Olenyuk, P. J. Stang, Chem. Rev. 2000, 100, 853.
- 2H. S. Rzepa, Chem. Rev. 2005, 105, 3697.
- 3
- 3aD. Ajami, O. Oeckler, A. Simon, R. Herges, Nature 2003, 426, 819;
- 3bT. Kawase, M. Oda, Angew. Chem. 2004, 116, 4496;
10.1002/ange.200460050 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4396.
- 4O. Cador, D. Gatteschi, R. Sessoli, F. K. Larsen, J. Overgaard, A.-L. Barra, S. J. Teat, G. A. Timco, R. E. P. Winpenny, Angew. Chem. 2004, 116, 5308;
10.1002/ange.200460211 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 5196.
- 5D. M. Walba, R. M. Richards, R. C. Haltiwanger, J. Am. Chem. Soc. 1982, 104, 3219.
- 6
- 6aS. Tanda, T. Tsuneta, Y. Okajima, K. Inagaki, K. Yamaya, N. Hatakenaka, Nature 2002, 417, 397;
- 6bG. R. Patzke, Angew. Chem. 2003, 115, 1002;
10.1002/ange.200390227 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 972.
- 7
- 7aK.-H. Wong, K.-K. Cheung, M. C.-W. Chan, C.-M. Che, Organometallics 1998, 17, 3505;
- 7bC.-M. Che, C. K.-L. Li, R. W.-Y. Sun, S. C.-F. Kui, N. Zhu, Chem. Eur. J., DOI: .
- 8Prepared by treating 2,6-bis(2′-bromophenyl)pyridine with nBuLi, followed by addition of TMEDA; see: S. C. F. Kui, PhD dissertation, The University of Hong Kong, 2005 (see the Supporting Information for the X-ray crystal structure of 1 [9]).
- 9CCDC 294396–294400 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 10For other examples of metal complexes with the CNC dianion, see:
- 10aM. Maestri, C. Deuschel-Cornioley, A. von Zelewsky, Coord. Chem. Rev. 1991, 111, 117;
- 10bG. W. V. Cave, N. W. Alcock, J. P. Rourke, Organometallics 1999, 18, 1801;
- 10cW. Lu, M. C. W. Chan, K.-K. Cheung, C.-M. Che, Organometallics 2001, 20, 2477;
- 10dW. Lu, N. Zhu, C.-M. Che, Chem. Commun. 2002, 900;
- 10eV. W.-W. Yam, R. P.-L. Tang, K. M.-C. Wong, X.-X. Lu, K.-K. Cheung, N. Zhu, Chem. Eur. J. 2002, 8, 4066;
10.1002/1521-3765(20020902)8:17<4066::AID-CHEM4066>3.0.CO;2-O CAS PubMed Web of Science® Google Scholar
- 10fM. Polson, S. Fracasso, V. Bertolasi, M. Ravaglia, F. Scandola, Inorg. Chem. 2004, 43, 1950;
- 10gA. J. Wilkinson, A. E. Goeta, C. E. Foster, J. A. G. Williams, Inorg. Chem. 2004, 43, 6513;
- 10hJ. D. Crowley, I. M. Steele, B. Bosnich, Inorg. Chem. 2005, 44, 2989.
- 11Selected examples:
- 11aJ. C. Vickery, M. M. Olmstead, E. Y. Fung, A. L. Balch, Angew. Chem. 1997, 109, 1227;
10.1002/ange.19971091109 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 1179;
- 11bC. P. McArdle, M. J. Irwin, M. C. Jennings, R. J. Puddephatt, Angew. Chem. 1999, 111, 3571;
10.1002/(SICI)1521-3757(19991115)111:22<3571::AID-ANGE3571>3.0.CO;2-7 Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3376;10.1002/(SICI)1521-3773(19991115)38:22<3376::AID-ANIE3376>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 11cC. P. McArdle, J. J. Vittal, R. J. Puddephatt, Angew. Chem. 2000, 112, 3977;
10.1002/1521-3757(20001103)112:21<3977::AID-ANGE3977>3.0.CO;2-E Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3819;10.1002/1521-3773(20001103)39:21<3819::AID-ANIE3819>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 11dR. J. Puddephatt, Coord. Chem. Rev. 2001, 216–217, 313;
- 11eJ. H. K. Yip, J. Prabhavathy, Angew. Chem. 2001, 113, 2217;
10.1002/1521-3757(20010601)113:11<2217::AID-ANGE2217>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2159;10.1002/1521-3773(20010601)40:11<2159::AID-ANIE2159>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 11fF. Mohr, M. C. Jennings, R. J. Puddephatt, Angew. Chem. 2004, 116, 987;
10.1002/ange.200353127 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 969;
- 11gR. Lin, J. H. K. Yip, K. Zhang, L. L. Koh, K.-Y. Wong, K. P. Ho, J. Am. Chem. Soc. 2004, 126, 15852.
- 12
- 12aH. Schmidbaur, Chem. Soc. Rev. 1995, 24, 391;
- 12bH. Schmidbaur, Gold: Progress in Chemistry, Biochemistry, and Technology, Wiley, Chichester, 1999.
- 13Although it is possible to generate the P,P or M,M analogues of 4, no such structures were observed. Modeling studies revealed that the P,P or M,M structure renders all the phenyl groups of the two diphosphane ligands in an unfavorable eclipsed arrangement, in contrast with the favorable staggered arrangement of the phenyl groups of each diphosphane ligand in 4 (see the Supporting Information). The molecules of 4 in the crystal structure are linked by intermolecular CH⋅⋅⋅π interactions (a CH bond of a dppm phenyl group with the nearest dppm phenyl ring; closest H⋅⋅⋅C distances: 2.841 Å) to give 2D sheets.
- 14Further decrease in temperature resulted in freezing of the CDCl3 solvent, which, together with the poor solubility of 4 in other solvents, hampered NMR measurements at lower than −53 °C.
- 15No molecules of 5 were observed in the crystal structure of 4. The molecular symmetry of 4 (Ci) and 5 (C2h) would result in the appearance of two singlets and one singlet, respectively, in their 31P NMR spectra. The observation of only one singlet at 0 °C or above (see the Supporting Information) could arise from the rapid conformational changes depicted in Figure 3. Lowering the temperature to −40 °C causes the signals of 4 (δ=17.8, 14.7 ppm) and 5 (δ=17.0 ppm) to be resolved. The change from 4 to 5 can be effected by breaking the AuI⋅⋅⋅AuI interactions (note the longer AuI⋅⋅⋅AuI contact of 3.178(10) Å in 4 than that of 3.015(29)–3.040(29) Å in 3), accompanied by intramolecular rotations to render the phenyl groups of each Ph2PCCPPh2 ligand in an eclipsed arrangement. Therefore, 5 should be energetically less favored than 4, consistent with the increase in the amount of 4 but decrease in the amount of 5 upon lowering the temperature (see the Supporting Information).
- 16
- 16aC. F. Shaw III, Chem. Rev. 1999, 99, 2589;
- 16bM. J. McKeage, L. Maharaj, S. J. Berners-Price, Coord. Chem. Rev. 2002, 232, 127.