Stereoselective Synthesis of Oligo-α(2,8)-3-deoxy-D-manno-2-octulosonic Acid Derivatives
Graphical Abstract
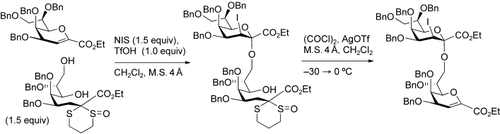
Iodoalkoxylation (see scheme) of a glycal with an acyclic saccharide precursor leads to an efficient stereoselective synthesis of di- and tri-α(2,8)-3-deoxy-D-manno-2-octulosonic acid (KDO; see picture). The glycal forms α-linked 3-iodo-KDO derivatives. The opening of the pyran ring improves the reactivity of the C8 hydroxy group. NIS=N-iodosuccimide, Tf=triflate, M.S.=molecular sieves