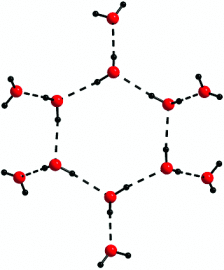
A Dodecameric Water Cluster Built around a Cyclic Quasiplanar Hexameric Core in an Organic Supramolecular Complex of a Cryptand†
Sujit K. Ghosh
Chemistry Department, Indian Institute of Technology Kanpur, Kanpur 208016, India, Fax: (+91) 512-259-7436
Search for more papers by this authorParimal K. Bharadwaj Prof. Dr.
Chemistry Department, Indian Institute of Technology Kanpur, Kanpur 208016, India, Fax: (+91) 512-259-7436
Search for more papers by this authorSujit K. Ghosh
Chemistry Department, Indian Institute of Technology Kanpur, Kanpur 208016, India, Fax: (+91) 512-259-7436
Search for more papers by this authorParimal K. Bharadwaj Prof. Dr.
Chemistry Department, Indian Institute of Technology Kanpur, Kanpur 208016, India, Fax: (+91) 512-259-7436
Search for more papers by this authorFinancial support from the Department of Science and Technology, New Delhi, India (grant No. SR/S5/NM-38/2003 to P.K.B.) and an SRF grant (CSIR) to S.K.G. are gratefully acknowledged.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z54002_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. Ludwig, Angew. Chem. 2001, 113, 1856;
10.1002/1521-3757(20010518)113:10<1856::AID-ANGE1856>3.0.CO;2-5 Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1808.10.1002/1521-3773(20010518)40:10<1808::AID-ANIE1808>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 2S. S. Xantheas, T. H. Dunning, Jr, J. Chem. Phys. 1993, 99, 8774.
- 3S. D. Colson, T. H. Dunning, Jr, Science 1994, 265, 43.
- 4K. Liu, J. D. Cruzan, R. J. Saykally, Science 1996, 271, 929.
- 5R. Ludwig, ChemPhysChem 2000, 1, 53.
- 6U. Buch, F. Huisken, Chem. Rev. 2000, 100, 3863.
- 7S. Maheswary, N. Patel, N. Sathyamurthy, A. D. Kulkarni, S. R. Gadre, J. Phys. Chem. A 2001, 105, 10 525.
- 8F. N. Keutsch, J. D. Cruzan, R. J. Saykally, Chem. Rev. 2003, 103, 2533.
- 9J. M. Ugalde, I. Alkorta, J. Elguero, Angew. Chem. 2000, 112, 733;
10.1002/(SICI)1521-3757(20000218)112:4<733::AID-ANGE733>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 2000, 39, 717.10.1002/(SICI)1521-3773(20000218)39:4<717::AID-ANIE717>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 10F. N. Keutsch, R. J. Saykally, Proc. Natl. Acad. Sci. USA 2001, 98, 10 533.
- 11M. Matsumoto, S. Saito, I. Ohmine, Nature 2002, 416, 409.
- 12J. L. Atood, L. J. Barbour, T. J. Ness, C. L. Raston, P. L. Raston, J. Am. Chem. Soc. 2001, 123. 7129.
- 13K. Nauta, R. E. Miller, Science 2000, 287, 293.
- 14L. J. Barbour, G. W. Orr, J. L. Atwood, Nature 1998, 393, 671.
- 15R. Custelcean, C. Afloroaei, M. Vlassa, M. Polverejan, Angew. Chem. 2000, 112, 3224;
10.1002/1521-3757(20000901)112:17<3224::AID-ANGE3224>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3094.10.1002/1521-3773(20000901)39:17<3094::AID-ANIE3094>3.0.CO;2-P CAS PubMed Web of Science® Google Scholar
- 16A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann, B. Botar, M. O. Talismanova, Angew. Chem. 2003, 115, 2131; Angew. Chem. Int. Ed. 2003, 42, 2085.
- 17K. M. Park, R. Kuroda, T. Iwamoto, Angew. Chem. 1993, 105, 939; Angew. Chem. Int. Ed. Engl. 1993, 32, 884.
- 18J. N. Moorthy, R. Natarajan, P. Venugopalan, Angew. Chem. 2002, 114, 3567;
10.1002/1521-3757(20020916)114:18<3567::AID-ANGE3567>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 2002, 41, 3417.10.1002/1521-3773(20020916)41:18<3417::AID-ANIE3417>3.0.CO;2-B CAS PubMed Web of Science® Google Scholar
- 19A. Michaelides, S. Skoulika, E. G. Bakalbassis, J. Mrozinski, Cryst. Growth Des. 2003, 3, 487.
- 20W. B. Blanton, S. W. Gordon-Wylie, G. R. Clark, K. D. Jordan, J. T. Wood, U. Geiser, T. J. Collins, J. Am. Chem. Soc. 1999, 121, 3551.
- 21K. Raghuraman, K. K. Katti, L. J. Barbour, N. Pillarsetty, C. L. Barnes, K. V. Katti, J. Am. Chem. Soc. 2003, 125, 6955.
- 22S. Pal, N. B. Sankaran, A. Samanta, Angew. Chem. 2003, 115, 1783; Angew. Chem. Int. Ed. 2003, 42, 1741.
- 23
- 23aJ. Kim, K. S. Kim, J. Chem. Phys. 1998, 109, 5886;
- 23bK. Kim, K. D. Jordan, T. S. Zwier, J. Am. Chem. Soc. 1994, 116, 11 568;
- 23cK. Liu, M. G. Brown, C. Carter, R. J. Saykally, J. K. Gregory, D. C. Clary, Nature 1996, 381, 501.
- 24
- 24aS. K. Ghosh, P. K. Bharadwaj, Inorg. Chem. 2003, 42, 8250;
- 24bC. Foces-Foses, F. H. Cano, M. Martinez-Ripoli, R. Faure, C. Roussel, R. M. Claramunt, C. Lopez, D. Sanz, J. Elguero, Tetrahedron: Asymmetry 1999, 10, 65.
- 25B. Kamb, Acta Crystallogr. 1964, 20, 1437.
- 26A. Rahaman, F. H. Stillinger, J. Am. Chem. Soc. 1973, 95, 7943.
- 27 Water and Biological Macromolecules (Ed.: ), CRC, Boca Raton, FL, 1993.
- 28S. Nishikiori, T. Iwamoto, J. Chem. Soc. Chem. Commun. 1993, 1555.
- 29C. J. Tsai, K. D. Jordan, J. Phys. Chem. 1993, 97, 5208.
- 30D. K. Chand, K Ragunathan, P. K. Bharadwaj, T. C. W. Mak, J. Org. Chem. 1996, 61, 1169.
- 31Crystal data for 1: Colorless, hexagonal, crystal dimensions 0.18×0.16×0.13 mm, trigonal, space group R
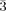 , Z=3, a=14.023(5), b=14.023 (5), c=33.249(5) Å, V=5662.3(2) Å3, ρcalcd=0.199 g cm−3, T=100 K, μ=0.015 mm−1, θmax=28.32°, 3139 reflections collected of which 2834 were unique. R1=0.0684, wR2=0.2004 with a GOF of 1.050 for I>2σ(I); residual electron density: 1.347 and −0.854 e Å−3. The X-ray data were collected on a Bruker SMART APEX CCD diffractometer using graphite monochromated MoKα radiation (0.71069 Å). The following programs were used during data collection and refinement: SMART (version 5.628 to acquire frame data), SAINT (version 6.45 to determine final unit parameters and intregate frame data). The structure was solved by direct methods and refined on F2 using SHELX-97 (G. M. Sheldrick, Program for the solution and refinement of crystal structures, University of Göttingen, Germany, 1997) package. The hydrogen atoms connected to the OW3 could not be located in the difference Fourier maps with certainty although other H atoms could be located. The non-hydrogen atoms were refined anisotropically. H atoms were not refined. CCDC-230380 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
, Z=3, a=14.023(5), b=14.023 (5), c=33.249(5) Å, V=5662.3(2) Å3, ρcalcd=0.199 g cm−3, T=100 K, μ=0.015 mm−1, θmax=28.32°, 3139 reflections collected of which 2834 were unique. R1=0.0684, wR2=0.2004 with a GOF of 1.050 for I>2σ(I); residual electron density: 1.347 and −0.854 e Å−3. The X-ray data were collected on a Bruker SMART APEX CCD diffractometer using graphite monochromated MoKα radiation (0.71069 Å). The following programs were used during data collection and refinement: SMART (version 5.628 to acquire frame data), SAINT (version 6.45 to determine final unit parameters and intregate frame data). The structure was solved by direct methods and refined on F2 using SHELX-97 (G. M. Sheldrick, Program for the solution and refinement of crystal structures, University of Göttingen, Germany, 1997) package. The hydrogen atoms connected to the OW3 could not be located in the difference Fourier maps with certainty although other H atoms could be located. The non-hydrogen atoms were refined anisotropically. H atoms were not refined. CCDC-230380 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 32See Supporting Information.
- 33C. J. Gruenloh, J. R. Carney, C. A. Arrington, T. S. Zwier, S. Y. Fredericks, K. D. Jordan, Science 1997, 276, 1678.
- 34G. A. Jeffrey, An Introduction to Hydrogen Bonding I, Oxford University Press, Oxford, 1997.
- 35D. Eisenberg, W. Kauzmann, The Structure and Properties of Water, Oxford University Press, Oxford, 1969.
- 36J. D. Joannopoulous, Nature 2001, 414, 257.
- 37P. R. ten Wolde, D. Frenkel, Science 1997, 277, 1975.