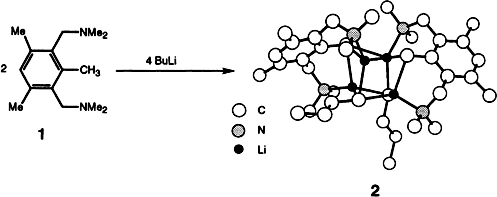
The Formation of a Mixed Organolithium Aggregate Li4R2nBu2 during the Heteroatom-Assisted Lithiation of 1,3-Bis(dimethylaminomethyl)-2,4,6-trimethylbenzene (R = 2,6-(CH2NMe2)2-3,5-Me2C6HCH2)†
Peter Wijkens
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Search for more papers by this authorErnout M. van Koten
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Search for more papers by this authorMaurits D. Janssen
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Search for more papers by this authorDr. Johann T. B. H. Jastrzebski
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Search for more papers by this authorDr. Anthony L. Spek
Bijvoet Center for Biomolecular Research Laboratory of Crystal and Structural Chemistry, Utrecht University
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerard van Koten
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615Search for more papers by this authorPeter Wijkens
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Search for more papers by this authorErnout M. van Koten
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Search for more papers by this authorMaurits D. Janssen
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Search for more papers by this authorDr. Johann T. B. H. Jastrzebski
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Search for more papers by this authorDr. Anthony L. Spek
Bijvoet Center for Biomolecular Research Laboratory of Crystal and Structural Chemistry, Utrecht University
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerard van Koten
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615
Debye Institute, Department of Metal-Mediated Synthesis, Utrecht University, Padualaan 8, NL-3584 CH Utrecht (The Netherlands) Telefax: Int. code +(30) 523-615Search for more papers by this authorDedicated to Professor Ekkehard Lindner on the occasion of his 60th birthday
Graphical Abstract
References
- 1 G. van Koten, Pure Appl. Chem. 1989, 61, 1681.
- 2 G. van Koten, Pure Appl. Chem. 1990, 62, 1155.
- 3 A. de Koster, J. A. Kanters, A. L. Spek, A. A. H. van der Zeijden, G. van Koten, K. Vrieze, Acta Crystallogr. Sect. C 1985, 41, 893.
- 4 J. A. M. van Beek, G. van Koten, W. J. J. Smeets, A. L. Spek, J. Am. Chem. Soc. 1986, 108, 5010.
- 5 D. M. Grove, G. van Koten, A. H. M. Verschuren, J. Mol. Catal. 1988, 45, 169.
- 6 H. W. Gschwend, H. R. Roderiguez, Org. React. 1979, 26, 1.
- 7 J. T. B. H. Jastrzebski, G. van Koten, Inorg. Synth. 1989, 26, 150.
- 8 With this knowledge in mind, it is obvious that reaction of 1 with 2 equiv of n BuLi would afford 2 in quantitative yield (see Experimental Procedure).
- 9
X-ray crystal structure data for 2: C38H68Li4N4, monoclinic, space group P21/c, a = 10.8476(7), b = 16.5638(11), c = 22.3064(12) Å, β = 101.525(5)°, V = 3927.1(4) Å3, Pcalcd = 1.030 gcm−3, μ(MoKα) = 0.5 cm−1, Z = 4, 8946 unique reflections (1.5 < θ < 27.4), 3800 with F0 > 4.0σ(F0). Enraf-Nonius CAD4T rotating anode diffractometer, graphite monochromated MoKα radiation, λ = 0.7 1073 Å, T = −123 °C. Solution by direct methods (SHELXS86), refinement of F2 with SHELXL-93 converged at R1 (wR2) = 0.088 (0.242), w = 1/(σ2(F
 ) + (0.1124P)2) for 416 refined parameters (anisotropic temperature factors for the nonhydrogen atoms). Hydrogen atoms were included at calculated positions (CH = 0.98 Å), riding on their carrier atoms. Further details of the crystal structure investigation are available on request from the Cambridge Crystallographic Data Centre, University Chemical Laboratory, 12 Union Road, Cambridge CB2 1EZ (UK), on quoting the full journal citation.
) + (0.1124P)2) for 416 refined parameters (anisotropic temperature factors for the nonhydrogen atoms). Hydrogen atoms were included at calculated positions (CH = 0.98 Å), riding on their carrier atoms. Further details of the crystal structure investigation are available on request from the Cambridge Crystallographic Data Centre, University Chemical Laboratory, 12 Union Road, Cambridge CB2 1EZ (UK), on quoting the full journal citation.
- 10 The two benzylic and the two butyl groups are crystallographically inequivalent and afford slightly but not significantly different bond lengths and angles.
- 11 S. P. Patterman, I. L. Karle, G. D. Stucky, J. Am. Chem. Soc. 1970, 92, 1150.
- 12 M. A. Beno, H. Hope, M. M. Olmstead, P. P. Power, Organometallics 1985, 4, 2117.
- 13 W. Zarges, M. Marsch, K. Harms, G. Boche, Chem. Ber. 1989, 122, 2303.
- 14 W. Zarges, M. Marsch, K. Harms, W. Koch, G. Frenking, G. Boche, Chem. Ber. 1991, 124, 543.
- 15 E. Weiss, Angew. Chem. 1993, 105, 1565. Angew. Chem. Int. Ed. Engl. 1993, 32, 1501.
- 16 G. W. Klumpp, Recl. Trav. Chim. Pays-Bas 1986, 105, 1.
- 17 J. T. B. H. Jastrzebski, G. van Koten, M. Konijn, C. H. Stam, J. Am. Chem. Soc. 1982, 104, 5490.
- 18 H. Köster, D. Thoenes, E. Weiss, J. Organomet. Chem. 1978, 160, 1.
- 19 H. Hope, P. P. Power, J. Am. Chem. Soc. 1983, 105, 5320.
- 20 The kinetic intermediates are most likely smaller aggregates. The tetranuclear lithium aggregates are the thermodynamically most stable species.
- 21 G. van Koten, J. T. B. H. Jastrzebski, Tetrahedron 1989, 45, 569.
- 22
However, in the case of the present benzylic diamine chelate the \documentclass{article}\usepackage{amssymb}\pagestyle{empty}\begin{document}${\rm Li}_4{\rm Bu}_2({\mathop{\rm C\,N}\limits^{\frown}}_2)_2$\end{document}
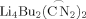 aggregate is further stabilized, since the two \documentclass{article}\usepackage{amssymb}\pagestyle{empty}\begin{document}${\mathop{\rm C\,N}\limits^{\frown}}_2$\end{document}
aggregate is further stabilized, since the two \documentclass{article}\usepackage{amssymb}\pagestyle{empty}\begin{document}${\mathop{\rm C\,N}\limits^{\frown}}_2$\end{document}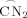 groups effectively cap the four Li atoms and prevent further attack by the free benzylic diamine ligands.
groups effectively cap the four Li atoms and prevent further attack by the free benzylic diamine ligands.
- 23 I. C. M. Wehman-Ooyevaar, G. M. Kapteijn, D. M. Grove, A. L. Spek, G. van Koten, J. Organomet. Chem. 1993, 452, C1.
- 24 Obtained in almost quantitative yield by reaction of 1,3-bis(bromomethyl)-2,4,6-trimethylbenzene [25] with 4 equiv of Me2NH: characterized by 1H and 13C NMR spectroscopy and GCMS.
- 25 A. W. van der Made, R. H. van der Made, J. Org. Chem. 1993, 58, 1262.
- 26 Detailed 1H, 13C, and 6Li NMR data are available upon request from the authors.