Pentadienyl, a More Reactive and More Strongly Bound Ligand Than Cyclopentadienyl†
This work was supported by the National Science Foundation (USA).
Graphical Abstract
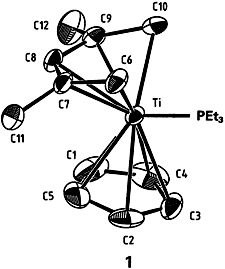
Direct comparison of the cyclopentadienyl ligand with its open-chain counterpart has been accomplished using complex 1, which contains both ligands. The Ti-C bonds to the acyclic ligand are 0.106 Å shorter than those to the cyclic ligand. Although this finding indicates that the bonds to the pentadienyl ligand are stronger, reaction of 1 with acetonitrile occurs nonetheless at this ligand. The aromatic character of C5H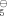 offers a possible explanation for this apparent contradiction. The aromaticity results in resistance to chemical change; that is, both formation of stronger bonds and coupling with electrophiles are disfavored.
offers a possible explanation for this apparent contradiction. The aromaticity results in resistance to chemical change; that is, both formation of stronger bonds and coupling with electrophiles are disfavored.