Novel Fulvalene Derivatives of Zirconium: A Facile Entry into Organozirconium(III) Chemistry†
Dr. Terence V. Ashworth
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Search for more papers by this authorDr. Tomas Cuenca Agreda
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Search for more papers by this authorDr. Eberhardt Herdtweck
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wolfgang A. Herrmann
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)Search for more papers by this authorDr. Terence V. Ashworth
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Search for more papers by this authorDr. Tomas Cuenca Agreda
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Search for more papers by this authorDr. Eberhardt Herdtweck
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Wolfgang A. Herrmann
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)
Anorganisch-chemisches Institut der Technischen Universität München, Lichtenbergstrasse 4, D-8046 Garching (FRG)Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft, the Bundesministerium für Forschung und Technologie, the Alexander-von-Humboldt Foundation (Fellowships to T. V. A. and T. C. A.), and Hoechst Aktiengesellschaft.
Graphical Abstract
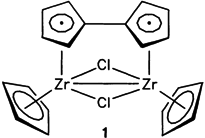
A universally modifiable new starting substance, the fulvalene ZrIII complex 1, is formed upon reaction of Cp2ZrCl2 and sodium amalgam. 1 is highly reactive, but nevertheless it can be isolated. The fulvalene ligand ensures that the secondary products are dinuclear, e.g. [(CpZrCl)2(C5H4−C5H4)O], the oxidation product of 1.
References
- 1 K. I. Gell, T. V. Harris, J. Schwartz, Inorg. Chem. 20 (1981) 481.
- 2 K. I. Gell, J. Schwartz, J. Am. Chem. Soc. 103 (1981) 2687; G. Fochi, G. Guidi, C. Floriani, J. Chem. Soc. Dalton Trans. 1984, 1253; T. Cuenca, P. Royo, J. Organomet. Chem. 293 (1985) 61; S. R. Wade, M. G. H. Wallbridge, G. R. Willey, J. Chem. Soc. Dalton Trans. 1983, 2555
- 3
G. P. Pez,
J. N. Armor,
Adv. Organomet. Chem.
19
(1981) 1;
M. Bottrill,
P. D. Gavens,
J. McMeeking in
G. Wilkinson,
F. G. A. Stone,
E. W. Abel (Eds.):
Comprehensive Organometallic Chemistry, Vol. 3, Pergamon, Oxford,
1982, p. 281.
10.1016/B978-008046518-0.00031-3 Google Scholar
- 4Titanium complexes of fulvalenes: (a) H. H. Brintzinger, J. E. Bercaw, J. Am. Chem. Soc. 92 (1970) 6182; (b) A. Davison, S. S. Wreford, J. Am. Chem. Soc. 96 (1974) 3017; (e) L. J. Guggenberger, F. N. Tebbe, J. Am. Chem. Soc. 95 (1973) 7870; (d) J. Am. Chem. Soc. 98 (1976) 4137; (e) J. J. Salzmann, P. Mosiman, Helv. Chim. Acta 50 (1976) 1831; (f) G. J. Olthof, J. Organomet. Chem. 128 (1977) 367; (g) E. G. Perevalova, I. F. Urazowski, D. A. Lemenovskii, Y. L. Slovokhotov, Y. T. Struchkov, J. Organomet. Chem. 289 (1985) 319.
- 5 D. J. Cardin, M. F. Lappert, C. L. Raston, P. I. Riley in G. Wilkinson, F. G. A. Stone, E. W. Abel (Eds.): Comprehensive Organometallic Chemistry Vol. 3, Pergamon, Oxford, 1982, p. 606.
- 6 A structure analogous to 2 has been proposed for [{ZrI(C5H4)(ν5-C5H5)} n] (n>2),[1] although no mass spectrum could be obtained, and cryoscopic molecular weight studies suggested a concentration-dependent oligomerization.
- 7 Experimental: A solution of 1 (1.17 g, 4 mmol) in toluene (50 mL) was treated with 1% Na/Hg (0.138 g Na, 6 mmol) and stirred for 12 h under nitrogen. The resultant dark red reaction mixture was then heated under reflux for 8 h. After filtration, evaporation, and recrystallization from toluene/n-hexane, the product was obtained as a dark red air-sensitive powder. Yield: 0.76 g (75%). An analytically pure sample was obtained by vacuum sublimation (10−5 torr). At ca. 120°C the oxo derivative 3 sublimed and pure 2 was obtained at 190°C.–Spectra: EI-MS: 2, M θ 508; 3, M θ 524; both molecular ions exhibit an isotopic pattern typical of an ion containing a Zr2CI2 moiety.-IR of 3 (KBr, v̈(ZrOZr). cm−1): 735 sand 702 s.-NMR (270 MHz, C6D6, 34°C): 2: 1H: δ = 5.57 (s, 10 H, C5H5), 4.93 (pseudotriplet, 4 H), and 3.97 (pseudotriplet, 4 H). The pseudotriplets arise from an AA′BB′ spin system with Jexp. ≈ JAB ≈ 2.7 Hz.- 13C: δ = 104.7 (C5H5), 104.6 and 101.1 (C2, C3), 106.2 (Cl). -3 1H (270 MHz, CDCI3, + 28°C): δ = 6.27 (s, 10 H, C5H5), 6.75 (m*, 2 H), 6.32 (m*, 2 H); 5.97 (m*, 2 H); m* = multiplets of an ABCD system.-13C: δ = 114.2 (C5H5), 104.2, 111.8, 113.7, 114.6 (C2-5), Cl not observed.
- 8 B. A. Frenz et al.: SDP, Structure Determination Package. College Station, TX 77840 (USA). 3, pale yellow crystals (from toluene); V = 2583.9 × 106 pm3, C2/c, Z = 4, F000 = 794, p = 1.59 g cm−3, μ = 10.2 cm−1; CAD-4 (Enraf Nonius), Mokα, graphite monochromator, ω-scan (Δω = 0.80°tgθ, 2° ≤θ≤22°), tmax = 60 s, h(–16/16), k(0/13), l(–15/15), 1471 reflections (I>1σ(I)).–Solution of structure: Patterson and difference Fourier methods; all non-hydrogen atoms refined anisotropically; H-atoms calculated from ideal geometry, included in structure factor calculations, but not refined; R = 0.046, Rw = 0.055.— Further details of the crystal structure determination are available on request from the Fachinformationszentrum Energie, Physik, Mathematik GmbH, D-7514 Eggenstein-Leopoldshafen 2, on quoting the depository number CSD-51 661, the names of the authors, and the full citation of the journal.
- 9 S. Motherwell, PLUTO, Program for Plotting Molecules and Crystal Structures, Cambridge (UK).
- 10 J. F. Clarke, M. G. B. Drew, Acta Crystallogr. B 30 (1974) 2267.
- 11 J. L. Peterson, J. Organomet. Chem. 166 (1979) 179, and references therein.
- 12 K. P. C. Vollhardt, T. W. Weidman, Organometallics 3 (1984) 82; J. C. Smart, C. J. Curtis, Inorg. Chem. 16 (1977) 1788, and references therein.