Porphycene—a Novel Porphin Isomer†
Corresponding Author
Prof. Dr. Emanuel Vogel
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)Search for more papers by this authorMatthias Köcher
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)
Search for more papers by this authorDr. Hans Schmickler
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)
Search for more papers by this authorDr. Johann Lex
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Emanuel Vogel
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)Search for more papers by this authorMatthias Köcher
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)
Search for more papers by this authorDr. Hans Schmickler
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)
Search for more papers by this authorDr. Johann Lex
Institut für Organische Chemie der Universität, Greinstrasse 4, D-5000 Köln 41 (FRG)
Search for more papers by this authorThis work was supported by the Stiftung Volkswagenwerk and the Fonds der Chemischen Industrie.
Graphical Abstract
References
- 1 J. F. M. Oth, H. Baumann, J.-M. Gilles, G. Schröder, J. Am. Chem. Soc. 94 (1972) 3498.
- 2(a) H. H. Inhoffen, J. W. Buchler, P. Jäger, Fortschr. Chem. Org. Naturst. 26 (1968) 284; (b) H. Scheer, J. J. Katz in K. M. Smith: Porphyrins and Metalloporphyrins, Elsevier, Amsterdam, 1975, p. 405; (c) T. R. Janson, J. J. Katz in D. Dolphin: The Porphyrins, Vol. IV, Academic Press, New York, 1979, p. 1.
- 3 The structural analysis of porphin 2, according to which the NH hydrogen atoms are localized on two pyrrole rings that are situated diagonally to each other, indicates that the outer 18π-electron pathway of conjugation is preferred. B. M. L. Chen, A. Tulinsky, J. Am. Chem. Soc. 94 (1972) 4144.
- 4The UV/VIS spectra of octaethylporphinatomagnesium and 12⊖ are almost identical: J.-H. Fuhrhop, Angew. Chem. 86 (1974) 363; Angew. Chem. Int. Ed. Engl. 13 (1974) 321.
- 5(a) D. Tanner, O. Wennerström, E. Vogel, Tetrahedron Lett. 23 (1982) 1221; (b) E. Vogel, U. Kürschner, H. Schmickler, J. Lex, O. Wennerström, D. Tanner, U. Norinder, C. Krüger, Tetrahedron Lett. 26 (1985) 3087; (c) K. Müllen, T. Meul, E. Vogel, U. Kürschner, H. Schmickler, O. Wennerström, Tetrahedron Lett. 26 (1985) 3091.
- 6(a) Porphyrins with various heteroatoms: A. W. Johnson in K. M. Smith (Ed.): Porphyrins and Metalloporphyrins, Elsevier, Amsterdam, 1975, p. 729; (b) homoporphyrins: H. J. Callot, E. Schaeffer, J. Org. Chem. 42 (1977) 1567; (c) sapphyrins: V. J. Bauer, D. L. J. Clive, D. Dolphin, J. B. Paine III, F. L. Harris, M. M. King, J. Loder, S.-W. Chien Wang, R. B. Woodward, J. Am. Chem. Soc. 105 (1983) 6429; (d) pentaphyrin: H. Rexhausen, A. Gossauer, J. Chem. Soc. Chem. Commun. 1983, 275; (e) platyrins: E. LeGoff, R. A. Berger, O. G. Weaver, Abstr. 10th Int. Congr. Heterocycl. Chem., Waterloo, Canada 1985.
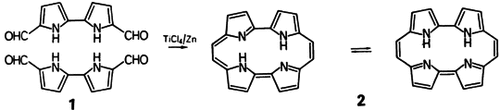
- 7 The name porphycene is suggested for 4, because this compound possesses structural features of both porphyrins and acenes. The solubility and chromatographic behavior indicates that 4 is less polar than 2 and thus more similar to hydrocarbons.
- 8(a) T. Mukaiyama, T. Sato, J. Hanna, Chem. Lett. 1973, 1041; (b) J. E. McMurry, M. P. Fleming, J. Am. Chem. Soc. 96 (1974) 4708; J. E. McMurry, Acc. Chem. Res. 16 (1983) 405; (c) D. Lenoir, Synthesis 1977, 553; (d) an example for the use of the McMurry reaction in porphyrin chemistry was recently reported: G. Holze, H. H. Inhoffen, Angew. Chem. 97 (1985) 887; Angew. Chem. Int. Ed. Engl. 24 (1985) 867.
- 9 The signal of the NH protons remain unchanged too −110°C (CD2Cl2/CF2Br2, 1:1), the lowest temperature achieved so far. 4 undergoes H/D exchange only very slowly in THF/D2O. For this reason, the N-deuterated compound may be prepared most practically by successive treatment of 4 with butyllithium (in ether) and D2O.
- 10
H. Budzikiewicz in
D. Dolphin:
The Porphyrins. Vol. III,
Academic Press, New York,
1978, p. 395.
10.1016/B978-0-12-220103-5.50016-7 Google ScholarA detailed mass spectrometric study of 4 and its derivatives is being carried out with Prof. H. Budzikiewicz (Universität Köln).
- 11 High-resolution mass spectra and elemental analysis are correct.
- 12 IR spectrum (KBr) of 4. v = 3099, 1562, 1469, 1410, 1224, 1167, 1054, 932, 813, 745, and 651 cm−1. An evaluation of the IR spectrum by means of N-deuterated 4 is in progress.
- 13 4 forms monoclinic crystals, space group P21/c, a = 10.088(3), b = 4.987(1), c = 15.464(4) Å, β = 108.36(2)°, Z = 2; 710 reflections, R = 0.030. Further details of the crystal structure investigation may be obtained from the Fachinformationszentrum Energie, Physik, Mathematik GmbH, D-7514 Eggenstein-Leopoldshafen 2 (FRG), on quoting the depository number CSD-51701, the names of the authors, and the journal citation.
- 14Remarkably, expansion of CCC angles on this order of magnitude is observed even for maleic acid. M. N. G. James, G. J. B. Williams, Acta Crystallogr. B30 (1974) 1249.
- 15A similarly short NN distance (2.626 Å) has also been found for the hydroperchlorate salt of the “proton sponge” 4,5-bis(dimethylamino)fluorene. H. A. Staab, T. Saupe, C. Krieger, Angew. Chem. 95 (1983) 748: Angew. Chem. Int. Ed. Engl. 22 (1983) 731.
- 16 We thank Prof. O. Ermer (Universität Köln) for helpful discussions in, the interpretation of the X-ray structure data of 4.
- 17 According to investigations of Dr. K. Pramod (note added in proof, January 22, 1986): 4 has also been converted into the zinc, copper, and cobalt complexes.