Mirror-Image Random Nonstandard Peptides Integrated Discovery (MI-RaPID) Technology Yields Highly Stable and Selective Macrocyclic Peptide Inhibitors for Matrix Metallopeptidase 7
Abstract
Matrix metallopeptidase 7 (MMP7) plays a crucial role in cancer metastasis and progression, making it an attractive target for therapeutic development. However, the development of selective MMP7 inhibitors is challenging due to the conservation of active sites across various matrix metalloproteinases (MMPs). Here, we have developed mirror-image random nonstandard peptides integrated discovery (MI-RaPID) technology to discover innate protease-resistant macrocyclic peptides that specifically bind to and inhibit human MMP7. One identified macrocyclic peptide against D-MMP7, termed D20, was synthesized in its mirror-image form, D’20, consisting of 12 D-amino acids, one cyclic β-amino acid, and a thioether bond. Notably, it potently inhibited MMP7 with an IC50 value of 90 nM, and showed excellent selectivity over other MMPs with similar substrate specificity. Moreover, D’20 inhibited the migration of pancreatic cell line CFPAC-1, but had no effect on the cell proliferation and viability. D’20 exhibited excellent stability in human serum, as well as in simulated gastric and intestinal fluids. This study highlights that MI-RaPID technology can serve as a powerful tool to develop in vivo stable macrocyclic peptides for therapeutic applications.
Introduction
The random nonstandard peptides integrated discovery (RaPID) system, which consists of a flexible in vitro translation (FIT) system and mRNA display technology, is one of the most powerful methods for selecting de novo macrocyclic peptide binders to target proteins of interest.1-4 The FIT system includes an artificial tRNA-aminoacylating ribozyme called flexizyme,5 which can charge tRNA with various non-proteinogenic amino acids, including β-amino acids6 and D-amino acids.5-8 β-amino acids have demonstrated the ability to modulate peptide conformations, while β-amino acid-containing peptides have shown better serum stability compared with those consisting solely of α-amino acids.9-11 Peptides composed entirely of D-amino acids are highly resistant against protease digestion; thus, they have a longer half-life and a lower immunogenicity than their L-counterparts.12, 13 The high diversity of the RaPID library, exceeding 1012 members of macrocyclic peptides, has led to the discovery of potent molecules that bind to various protein targets through affinity selection.14-18 However, the incorporation of consecutive D-amino acids has proven to be challenging. Suga and co-workers successfully constructed a hybrid L/D RaPID library with a maximum of five D-amino acids incorporated into a single macrocyclic peptide.19 In order to develop macrocyclic peptides that are mainly composed of D-amino acids, we decided to develop the mirror-image RaPID (MI-RaPID) technology, analogous to the mirror-image phage display (MIPD) developed by Kim and co-workers in the mid-90s.20 In this approach, the RaPID system is used to perform affinity selection against the mirror-image form of the target protein (composed of D-amino acids and achiral glycine). The mirror-image forms of the identified macrocyclic peptide binders are subsequently synthesized and used to target the corresponding L-protein (Figure 1). This strategy holds great potential for developing highly stable macrocyclic therapeutic peptides targeting overexpressed proteins in cancer cells (and other pathologies) such as MMPs.21
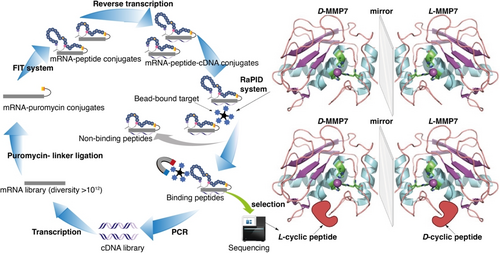
Mirror-image RaPID (MI-RaPID) system against MMP7.
MMPs, a family of multidomain zinc-dependent endopeptidases, have been reported to hydrolyze components of the extra-cellular matrix (ECM), thereby inducing tissue remodeling.22 MMPs share three common domains: the pro-domain, the zinc-dependent catalytic domain, and the hemopexin-like C-terminal domain.23 MMPs are typically expressed as inactive precursors (zymogens) that contain the pro-domain, which is removed upon activation.24 To date, the mechanisms of MMP activation are not fully understood. The activity of MMPs is tightly regulated by their endogenous inhibitors, such as tissue inhibitors of metalloproteinases (TIMPS). Failure to maintain a balance between the activity of MMPs and their inhibitors has been widely recognized as a contributor to pathological conditions such as cancer cell invasion and metastasis.25-27 To date, at least 23 different metalloproteinases have been identified in humans. These MMPs are divided into six subclasses based on their substrate specificity: collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs.26, 28 This study focuses on MMP7 (matrilysin).
MMP7, the smallest MMP, plays an important role in various physiological and pathological processes.29 Previous studies have reported that MMP7 hydrolyzes a range of ECM substrates and non-ECM proteins. Identified substrates of MMP7 include laminin, fibronectin and non-fibrillar collagen, gelatin, elastin, entactin/nidogen and tenascin-C,30 tumor necrosis factor α (TNF-α), Fas ligand, heparin-binding epidermal growth factor precursor (HB-EGF), β-integrin, and E-cadherin.31, 32 MMP7 is associated with human cancer invasion, apoptosis, growth, and angiogenesis.29 This protease has been found to be overexpressed in various cancers, including gastric, colorectal, lung, and liver cancers.33 The upregulation of MMP7 in cancer cells has been associated with tumor metastasis and poor survival rates in cancer patients.33-37 In recent years, the serum level of MMP7 has been widely recognized as a predictive biomarker for cancer progression in cancers such as pancreatic cancer and gastric cancer.38-41 Hence, MMP7 has emerged as a promising therapeutic target in cancer treatment. To date, a few peptide-based or small-molecule MMP7 inhibitors have been developed.42, 43 However, these MMP7 inhibitors show moderate selectivity over other MMPs and lack substantial stability. Therefore, there is a need to develop new selective and stable inhibitors to target MMP7.
In this study, we successfully produced a potent and stable MMP7 inhibitor with selectivity over other MMPs by using MI-RaPID technology. The cyclic peptide libraries containing β-amino acids were screened against the chemically synthesized biotinylated L-MMP7 and D-MMP7. Four cyclic peptides containing β-amino acids were identified as potential leads, two for each target. The selected cyclic peptides against D-MMP7 were synthesized in their mirror-image forms and examined by targeting the natural MMP7 using in vitro inhibitory assays. One examined cyclic peptide, composed of D-amino acids and a β-amino acid, targeted MMP7 with high potency (IC50=90 nM) and showed excellent selectivity over MMP1, MMP8, MMP9, MMP10 and MMP14. Additionally, this peptide variant demonstrated excellent stability in simulated gastric and intestinal fluids. These findings suggest that MI-RaPID technology can serve as a powerful tool to develop novel peptide-based inhibitors composed of D- and β-amino acids for various therapeutic targets.
Results and Discussion
MMP7 has attracted significant attention as an emerging therapeutic target in a range of cancers.33, 38-41 A recent study has reported that a monoclonal antibody against MMP7 (GSM-192) has been successfully developed and that this antibody inhibits MMP7 activity with an IC50 value of 132 nM. Moreover, this antibody was shown to induce cancer cell apoptosis, reduce cell migration, and increase cancer cell sensitivity toward chemotherapy, highlighting the potential of MMP7 in cancer treatment.60
Total Chemical Synthesis of the Biotinylated L- and D-Enantiomers of the Catalytic Domains of MMP7
The primary structure of the catalytic domain of MMP7 consists of 173 amino acids, MMP7(95–267) (MMP7cat, in short MMP7, Scheme 1a). Since this domain does not contain any cysteine residues, our synthetic approach was based on Fmoc-solid phase peptide synthesis (Fmoc-SPPS)44, 45 of four peptide segments. These segments were combined by three native chemical ligation (NCL) reactions at the Lys131-Ala132, His178-Ala179, and Ala211-Ala212 junctions (underlined, Scheme 1a).46 To facilitate the NCL reactions, we temporary substituted three Ala residues (A132, A179, and A212) with Cys or protected Cys in the form of Thz (Z), followed by a final desulfurization step.47, 48 While the corresponding C-terminal segment MMP7(212–267)(A212C) was synthesized by standard Fmoc-SPPS, the segments MMP7(179–211)(A179Z)-COSR, MMP7(132–178)(A132Z)-COSR, and the N-terminally biotinylated MMP7(95–131)-COSR (biotin is required for immobilization in RaPID) were prepared by Fmoc-SPPS as C-terminal thioester surrogates.49, 50
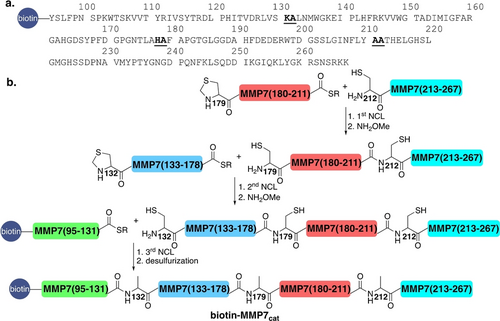
(a) The Sequence of the catalytic domain of MMP7. The NCL reaction junctions are underlined. (b) Schematic of the chemical synthesis approach for the catalytic domain of biotin-L- and D-MMP7, involving four segments and three NCL reactions, followed by desulfurization.
Upon the completion of the peptides’ syntheses and characterizations (details in the SI), MMP7(179–211)(A179Z)-COSR was ligated to the MMP7(212–267)(A212C) segment. Following ligation, the Thz group was opened with NH2OMe to yield Cys179, resulting in MMP7(179–267)(A179C, A212C) (18 mg, 35 % yield). Next, the purified MMP7(179–267)(A179C, A212C) was ligated with MMP7(132–178)(A132Z)-COSR, also featuring the N-terminal Thz group. After ligation and Thz opening, MMP7(132–267)(A132C, A179C, A212C) was isolated (10 mg, 36 % yield). For the final ligation, the purified MMP7(132–267)(A132C, A179C, A212C) was ligated to biotin-MMP7(95–131)-COSR, yielding biotin-MMP7(95–267)(A132C, A179C, A212C) (5 mg, 39 % yield). Subsequently, desulfurization produced 3 mg of biotinylated-L-MMP7 (60 % yield). Following the optimization of this synthetic route for biotinylated-L-MMP7, the same approach was used to prepare the biotinylated-D-MMP7 (with a final yield of 1.5 mg, see the Supporting Information for further details). The lower yield obtained for the D-proteins is likely due to the reduced purity of D-amino acid reagents.51, 52
After synthesis and purification (Figure 2a and 2b), both biotinylated-L–MMP7 and biotinylated-D-MMP7 were folded in 50 mM Tris-HCl buffer (pH 7.0), 10 mM CaCl2, 0.1 mM Zn acetate and 5 % v/v glycerol and left at 4 °C for 4 h.53 Subsequently, the structure of both proteins was analyzed by circular dichroism (CD). As expected, the biotinylated-D-MMP7 exhibited an inverted spectrum compared to the biotinylated-L-MMP7 (Figure 2c). The catalytic activity of biotinylated-L-MMP7 and recombinant MMP7 was determined by monitoring the hydrolysis of the fluorogenic peptide substrate FS-6 (λext=320 nm and λem=400 nm). Both exhibited similar activity profiles (Figure 2d).54, 55
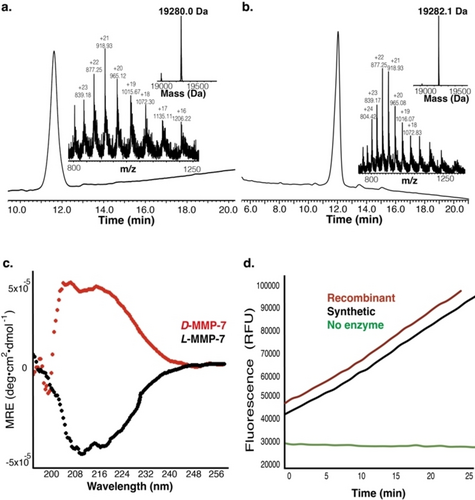
Characterization of biotin-L/D-MMP7cat. (a) HPLC and ESI-MS spectrum of biotin-L-MMP7cat; mass calculated: 19284.7 Da, observed: 19280.0±2.8 Da (b) HPLC and ESI-MS spectrum of biotin-D-MMP7cat; mass calculated: 19284.7 Da, observed: 19282.1±5.8 Da (c) CD spectra for 20 μM L- and D-MMP7cat. The biotin-D-MMP7cat exhibits an inverted spectrum of biotin-L-MMP7cat. (d) In vitro catalytic activity of synthetic and recombinant MMP7cat by monitoring the hydrolysis of the fluorogenic peptide substrate FS-6.
RaPID Selection Against Biotinylated L- and D-MMP7
RaPID technology has been broadly used to develop de novo macrocyclic peptide ligands for various proteins of interest. For instance, a RaPID library containing L,D-peptides was screened against human epidermal growth factor receptor (hEGFR), and two macrocyclic peptide binders were found to show good binding affinity to hEGFR.19 In another example, a macrocyclic peptide, HiP-8, identified by RaPID affinity selection, showed strong binding affinity (IC50=0.9 nM) towards hepatocyte growth factor (HGF).61 Moreover, four cyclic peptides (Ub2i, Ub2ii, Ub4i, and Ub4ix), which specifically bind to the K48-linked ubiquitin chain with Kd in the nanomolar range, were also developed based on the RaPID selection results.62, 63 Additionally, four macrocyclic peptides containing β-amino acids were developed based on RaPID affinity selection against human factor XIIa (hFXIIa) and demonstrated strong inhibitory activity with Ki in the nanomolar range.6 These findings suggest that the RaPID technology is a robust platform that can be used for drug discovery.
In this study, a cyclic peptide library containing three β-amino acids, namely (1S,2S)-2-ACHC, (1R,2S)-2-ACPC and (1R,2R)-2-ACPC, and two D-amino acids (ClAc-D-Tyr and D-Cys) was constructed as previously described (Figure 3a).6 The codons assigned for the three β-amino acids were AUC, CUC, and UGC. This assignment was chosen because these codons are less likely to encounter misincorporation in the FIT system. The codon for D-Cys was UGG, as this codon is known to offer high efficiency for incorporating D-Cys in the FIT system. Since ClAc-D-Tyr is the initial residue of the peptide translated, the codon chosen for this amino acid was AUG. To assess the incorporation efficiency of the non-proteinogenic amino acids in relation to the ribosome in the FIT system, we first prepared (1S,2S)-2-ACHC-tRNAPro1E2GAU, (1R,2S)-2-ACPC-tRNAPro1E2GAG, (1R,2R)-2-ACPC-tRNAGluE2GCA, D-Cys-tRNAPro1E2CCA, and ClAcD-Tyr-tNRAfMetCAU using the flexizymes. These pre-charged tRNAs were then added to the FIT system to translate three model peptides. Each model peptide contained one ClAc-D-Tyr at the N-terminus, one D-Cys at the C-terminus, and one β-amino acid in the middle of the sequence. The incorporation of the β-amino acid aimed at generating a turn structure in the peptide sequences. Two model peptides containing different 2-ACPC were translated without truncation, whereas the model peptide with 2-ACHC exhibited a big truncated peak (Figure S9a), indicating that the incorporation efficiency of 2-ACHC was low. To address this issue, we optimized the translation conditions by increasing the concentration of the pre-charged 2-ACHC-tRNA (from 25 μM to 40 μM), and the concentration of EF−Tu (from 20 μM to 70 μM). EF−Tu is an elongation factor that mediates the accommodation of charged tRNA into the ribosome A site. Using the optimized conditions, we successfully translated the 2-ACHC model peptide with no truncation (Figure S9b). These results indicated that the correct cyclic peptides could be achieved in this system.
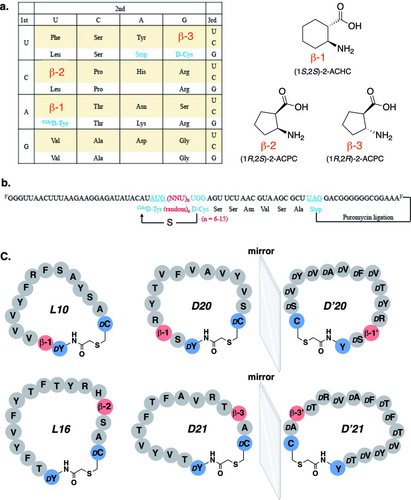
(a) Codon assignment for RaPID library construction and schematic of β-amino acids included in the library. (b) mRNA sequences and the corresponding peptide sequences of the constructed RaPID library. The mRNA and the resulting peptides are covalently linked by a puromycin linker. (c) Structures and sequences of the selected peptide binders (L10 and L16 from L-MMP7 RaPID selection, D20 and D21 from D-MMP7 RaPID selection). D’20 and D’21 are the mirror-image forms of D20 and D21. β-1’ represents (1R,2R)-2-ACHC and β-3’ represents (1S,2S)-2-ACPC, the mirror-image of β-1 and β-3, respectively.
Following a standard selection protocol,56 the translated peptide library with more than 1012 unique sequences was screened for affinity to the L- and D-MMP7 immobilized on streptavidin-coated magnetic beads. Here, the affinity selection results showed that the positive cDNA recovery percentage against L-MMP7 and D-MMP7 fluctuated across different selection rounds (Figure S10).
The increased recovery in round three of L- and D-MMP7 selections may be caused by the accumulation of primer dimers which appeared to be easier for PCR amplification, thus resulting in cDNA enrichment. The reason for the decreased recovery in round six of the D-MMP7 selection is still not clear. It could have occurred during the mRNA display selection round when cDNA enrichment was observed in the previous round. We halted the selection at round six.
To identify promising peptide binders for MMP7, we performed next-generation sequencing (NGS) on all cDNA, including the positive cDNA and negative cDNA obtained from each RaPID selection round. Overall, the random regions of the top enriched positive cDNA sequences targeting either L-MMP7 or D-MMP7 were not highly conserved but exhibited a preference for hydrophobic amino acids (e.g., Val and Ala, Figure S11). This result is consistent with a previous study regarding the substrate specificity of MMP7.57 MMP7 has a broad substrate specificity. Only the S′1 and S3 binding subsites of MMP7 show a preference for branched hydrophobic residues, including Leu, Ile and Val. Other binding subsites of MMP7, such as S4, S2–S1, and S′2 to S′4, show tolerance to various residues. The broad substrate specificity of MMP7 may explain the low convergence of the enriched cDNA sequencing results observed in this study.
Based on the NGS results, six positive cDNAs (L1, L2, L3, L4, L10 and L16) from the L-MMP selection and six positive cDNAs (D1, D2, D3, D6, D20 and D21) from the D-MMP selection were chosen for cloning to further verify their binding affinities towards MMP7. The reason for choosing these cDNA is that they all show a noticeably increasing trend in NGS reading percentages from selection round one to round six (Figure S12). The cloned cDNAs were translated into peptides and screened for affinity towards either L-MMP7 or D-MMP7 immobilized on streptavidin-coated magnetic beads. As shown in Table 1, among the L-MMP7 binders, L10 and L16 exhibited a good P/N (positivity recovery percentage vs. negative recovery percentage) ratio, and these two peptides appeared most frequently when targeting L-MMP7. Regarding the D-MMP7 binders, D6, D20, and D21 showed higher positivity recovery percentages, with the highest P/N ratio observed in D20, followed by D21 (Table 2). A good P/N ratio indicated that the binder indeed binds to the protein target rather than the streptavidin-coated magnetic beads. Based on these results, we selected L10, L16, D20, and D21 for further analysis.
Peptide |
Sequence |
Positive recovery % |
Negative recovery % |
P/N |
|---|---|---|---|---|
L1 |
AcDYTTYVFTFTYVFATSPDC |
0.01465 |
0.00244 |
6.0 |
L2 |
AcDYTVYVSFTYYYRTAHHDC |
0.01054 |
0.00894 |
1.2 |
L3 |
AcDYSVSVFRYYYFYYADC |
0.00534 |
0.01359 |
0.4 |
L4 |
AcDYTYTYVYYTVTYYSNVDC |
0.00200 |
0.00161 |
1.2 |
L10 |
AcDYβ1VVVYFRFSAYSADC |
0.05920 |
0.00094 |
63.2 |
L16 |
AcDYTFYVFYTFTYRHβ2SADC |
0.09358 |
0.00084 |
111.4 |
- All peptides are cyclized via a thioether bond between the N-terminal chloroacetamide group and the thiol group of cysteine. β1=(1S,2S)-2-ACHC, β2=(1R,2S)-2-ACPC.
Peptide |
Sequence |
Positive recovery % |
Negative recovery % |
P/N |
|---|---|---|---|---|
D1 |
AcDYTYTFYFYYTRFAFPDC |
0.01197 |
0.00271 |
4.4 |
D2 |
AcDYTFTYFYAVFSFRTDC |
0.14942 |
0.00210 |
71.1 |
D3 |
AcDYTTVYRVTYVYVSVDC |
0.01738 |
0.00043 |
40.3 |
D6 |
AcDYRYTYVYVSVSVVβ2DC |
0.37621 |
0.00798 |
47.1 |
D20 |
AcDYSβ1RYTVFVAVYVSDC |
0.29623 |
0.00030 |
975.6 |
D21 |
AcDYTVYVTFTFAVRTβ3ADC |
0.48196 |
0.00281 |
171.7 |
- All peptides are cyclized via a thioether bond between the N-terminal chloroacetamide group and the thiol group of cysteine. β1=(1S,2S)-2-ACHC, β2=(1R,2S)-2-ACPC, β3=(1R,2R)-2-ACPC.
Chemical Synthesis of Cyclic Peptide Binders
We synthesized L10, L16, and the mirror-image forms of D20 and D21, referred to as D’20 and D’21 (Figure 3). All peptides were synthesized by Fmoc-SPPS using rink amide resin, with a C-terminal solubility tag, polyethylene glycol PEG6 or PEG27, to enhance the solubility of these hydrophobic peptides.58 The synthesized peptides were chloroacetylated at the N-terminus with Cl−Ac-NHC. The peptides were then cleaved, and the linear peptides were treated with triethylamine (TEA) in DMSO to form a thioether bond between the N-terminal chloroacetamide group and the thiol group of the cysteine (Figure 4a). The respective peptides were isolated in milligram quantities and characterized by RP-HPLC and ESI-MS (Figure 4b–e). All these peptides showed good purity and correct masses.
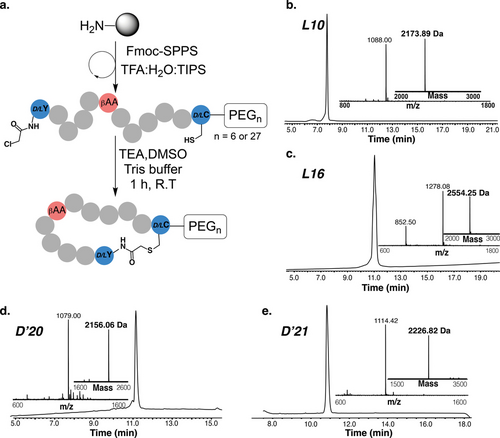
Synthesis and characterization of thioether-closed macrocyclic peptides. (a) Schematic of the synthesis of macrocyclic peptides using Fmoc-SPPS and the formation of the thioether bond; HPLC and ESI-MS spectrum for L10 (b), L16 (c), D’20 (d) and D’21 (e).
In Vitro Inhibition Activity Against MMP7
The activity of each cyclic peptide binder against MMP7 was assessed in competitive inhibition assays.55 Briefly, a serial dilution of each macrocyclic peptide (0–225 μM) was incubated with MMP7 (10 nM) for 30 min at 37 °C. The assay buffer consisted of 50 mM Tris-HCl pH 7.0, 100 mM NaCl, 5 mM CaCl2, 0.1 mM Zn acetate, and 0.05 % Brij35. The reaction was initiated by adding the fluorogenic substrate (FS-6) with a final concentration of 10 μM, and substrate cleavage was monitored for 1.5 h at λext=320 nm and λem=400 nm.55 The results showed that all four peptides inhibited the activity of MMP7 (Figure 5a, 5b, and Figure S14). By comparison, D’20 was the most potent MMP7 inhibitor, showing a biphasic inhibition behavior (IC50-1=90±13 nM, IC50-2=45±7 μM), followed by L16 (IC50-1=180 nM, IC50-2>100 μM), whereas L10 and D’21 exhibited weak activity against MMP7.
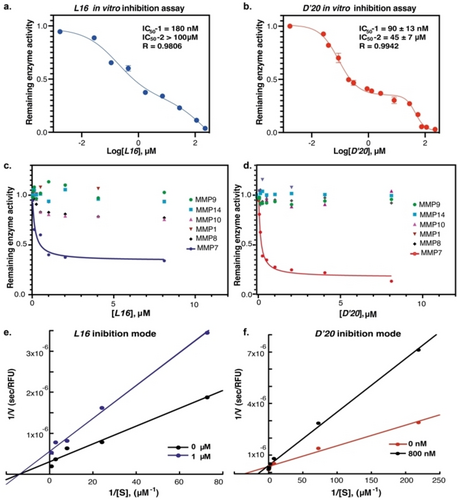
Dose-response curves for in vitro inhibition activity on human MMP7, which was examined using the fluorogenic peptide (FS-6). (a) L16 inhibited MMP7 with IC50-1=180 nM and IC50-2=290 μM. (b) D’20 inhibited MMP7 with IC50-1=90±13 nM and IC50-2=45±7 μM; Selectivity of (c) L16 and (d) D’20 over other MMPs (MMP1, MMP8, MMP9, MMP10, and MMP14); Double reciprocal plot of 1/V vs 1/[S] for (e) L16 and (f) D’20. All data points represent the mean±SD for each condition and n=3. The curves in (a) and (b) were fitted to the data using a biphasic model using Prism software with these equations: , where is the remaining activity of the enzyme and is the log inhibitor concentration.
The observed biphasic dose-response inhibition of MMP7 by L16 and D’20 may suggest dual high and low binding affinities, with the high-affinity binding mode accounting for approximately 65 % inhibition. We also tested the inhibition of MMP7 by D’20 using the HPLC method with a peptide substrate without the coumarin fluorophore. Using this method, we observed single-phase inhibition with an IC50 value of 71 nM (Figure S14c and S15), suggesting that the second inhibition phase observed might be due to nonspecific binding to coumarin fluorophore. Future studies will focus on better understanding this behavior through structural analysis of the L16/D’20-MMP7 complexes. Based on these results, D’20 and L16 were selected for further evaluation.
In Vitro Inhibition Activity Against other MMPs
To determine the selectivity of D’20 and L16, these two inhibitors were screened against other MMPs with similar substrate specificities, namely, MMP1, MMP8, MMP9, MMP10, and MMP14. The results showed that L16 inhibited MMP8 and MMP10 to some extent (Figure 5c), while D’20 was found to be highly selective over other MMPs (Figure 5d). The inhibitory activity of D’20 against other MMPs was at least 60-fold weaker compared to MMP7.
Determining the Mode of Inhibition
To determine the mode of inhibition of MMP7 by L16 and D’20, double reciprocal (Lineweaver–Burk) plots were obtained in the presence of various concentrations of the substrate FS-6, with or without 1 μM of L16 and 800 nM of D’20. For L16, the curves intersected at the x-axis, indicating non-competitive inhibition (Figure 5e), whereas D’20 exhibited the characteristics of competitive inhibition, showing unparallel lines with different slopes that intersect at the y-axis (Figure 5f).
Control Experiments with L16 and D’20 Analogs
To assess the stereospecificity of D’20 towards the inhibition of MMP7, we prepared D20, which has the same sequence as D’20, but is mainly composed of L-amino acids and β-1 (Table 2), and tested its inhibitory activity towards MMP7. The results revealed that the activity of MMP7 remained unaffected at concentrations as high as 28 μM of D20. This suggests that the inhibition of MMP7 is stereospecific and not due to unspecific hydrophobic interactions. We also examined scrambled D’20 (sc-D’20) and scrambled L16 (sc-L16). Neither sc-D’20 nor sc-L16 showed inhibition towards MMP7, indicating that the identified inhibitors in this study are highly specific (Figure S16).
In Vitro Stability Studies of L16 and D’20
The stability of L16 and D’20 was evaluated by monitoring their degradation in human serum, simulated gastric fluid (SGF), and simulated intestinal fluid (SIF) at 37 °C. SGF contains pepsin at pH 1.3, whereas SIF contains pancreatin at pH 6.8.59 At each time point (hours for D’20 and minutes for L16), a relative amount of the peptide samples was taken and checked by HPLC and MS. The results showed that both D’20 and L16 were stable in human serum for at least 24 h (Figure S17). Additionally, D’20 was stable in both SGF and SIF for at least 12 h, whereas L16 was completely degraded in SIF (<10 min) and in SGF (<30 min) (Figure 6).
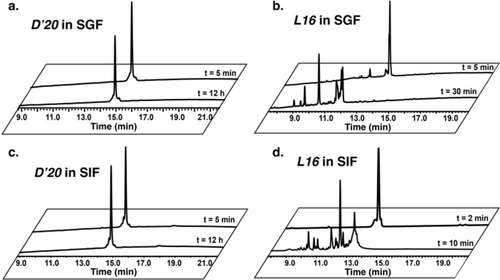
SGF and SIF stability of D’20 and L16. (a) HPLC spectrum of D’20 in SGF. (b) HPLC spectrum of L16 in SGF. (c) HPLC spectrum of D’20 in SIF. (d) HPLC spectrum of L16 in SIF.
Impact of Anti-MMP7 Macrocyclic Peptides on Cell Function
We decided to focus on the two most active macrocyclic peptides L16 and D’20, to study the impact of MMP7 inhibition on cell function. The pancreatic cell line CFPAC-1 expresses both zymogenic MMP7 and the activated enzyme, both of which are fully characterized for their MMP7 activity. To test the effect of macrocyclic peptide inhibition on pancreatic cancer cell proliferation and viability, 70 % confluent cultures were treated with various macrocyclic peptides. For the proliferation assay, cells were fixed every day for three days after drug treatment. For the viability assay, cells were fixed after 72 hours of drug treatment. Neither L16 nor D’20 showed any significant inhibition of proliferation or reduction in the viability of the CFPAC-1 cells (Figure S18). To study the impact on cell migration, CFPAC-1 cells were plated in transwell inserts at indicated drug dilutions, with fetal bovine serum in the bottom chambers to act as a chemoattractant. After 18 h, cells were fixed and stained with crystal violet solution. Migrating cells at the bottom of transwells were photographed, and the total number of migrating cells per image was determined. The results show that L16 did not have significant migration inhibition, while D’20 inhibited cell migration with an IC50 of about 1 μM (Figure 7). This IC50 is comparable to the effects observed with GSM-192, a highly selective anti-MMP7 inhibitory antibody.43
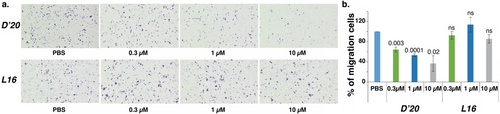
(a) Representative pictures of transwell migration after anti-MMP7 treatment with either D’20 or L16. (b) Bar plot represents the mean±SE of three biological replicates. Student's t-test p-values are reported.
Conclusions
In this project, we demonstrated the effectiveness of the MI-RaPID technology in the de novo discovery of potent peptide inhibitors for proteins of interest. We successfully developed a highly stable and selective cyclic peptide inhibitor composed predominantly of β- and D-amino acids for MMP7, which is a promising therapeutic target in various cancer types. First, we synthesized the catalytic domain of MMP7 in its L- and D-form using chemical protein synthesis. We then screened the L- and D-MMP7 in the RaPID system and identified several potential peptide binders for further development. Among these, D’20, synthesized as the mirror-image of the identified peptide (D20) binding to D-MMP7 in the selection, inhibited natural human MMP7 with an IC50 value in the nanomolar range. D’20 was found to be a selective inhibitor of MMP7, showing no cross-reaction with other sequentially and structurally similar MMPs, and demonstrating high stability in human serum, as well as in SIF and SGF. Remarkably, D’20 inhibited the migration of the pancreatic cell line CFPAC-1 without affecting cell proliferation and viability. These encouraging findings suggest that MI-RaPID facilitates the de novo discovery of stable and selective peptide inhibitors for proteins of interest, and represents a promising avenue for drug development against cancers and other diseases.
Supporting Information
Supporting Information and methods, including synthesis of peptides and proteins, characterization, RaPID for the target, synthesis of macrocyclic peptides, their characterization, mode of inhibition, and other relevant details, are available online. Additional references are cited within the Supporting Information.64-73
Acknowledgments
We thank the members of our respective laboratories for the fruitful discussions. H.G. thanks the British Council for GROWTH PhD Fellowship, and C.L. thanks the JSPS Grant-in-Aid for JSPS Fellows (23KF0020). J. M. S. acknowledges the support of the US-Israel Binational Science Foundation (BSF) 2017207, NIH R01CA258274, Israel Cancer Research Foundation (ICRF), Israel Science Foundation (3486/20) and the University of Toronto/HUJI research alliance in protein engineering. N. M. acknowledges the financial support of the Israel Science Foundation (1388/22). H.S. acknowledges the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Specially Promoted Research (JP20H05618). I.S. acknowledges the support of the European Union's Horizon 2020 research and innovation program (grant agreement No 801126), European Research Council (ERC, grant agreement No 695437), Israel Science Foundation (1800/19), the National Institutes of Health (NIH, grant No. 1R21AI146813), and The Thompson Family Foundation.
Conflict of Interests
H. Ghareeb, C. Y. Li, I. Sagi, H. Suga, and N. Metanis filled a provisional patent that covers this technology.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




