Calcium-Dependent Lipopeptide Antibiotics against Drug-Resistant Pathogens Discovered via Host-Dependent Heterologous Expression of a Cloned Biosynthetic Gene Cluster
Abstract
Historically, small molecules biosynthesised by bacteria have been an excellent source for antibacterial drugs. Today, however, the rediscovery of known compounds is a significant hurdle to developing new antimicrobials. Here we use a genome mining and synthetic biology approach to discover the ambocidins: calcium-dependent lipodepsipeptides that are active against drug-resistant Gram-positive pathogens. By cloning a silent biosynthetic gene cluster (the amb cluster) from Streptomyces ambofaciens ATCC 2387 and integrating this into the chromosome of Streptomyces avermitilis we induce expression of ambocidin A and B: two new Nϵ-hydroxyarginine-containing cyclic lipodepsipeptides active against drug-resistant Gram-positive pathogens. Using a panel of Streptomyces host strains, we show that the choice of heterologous host is critical for producing the biologically active compounds, and that inappropriate host choice leads to aberrant production inactive derivatives. We show that Nϵ-hydroxyarginine is the product of a heme-dependent oxygenase and that it enhances biological activity. Ambocidin A inhibits cell wall biosynthesis by binding to Lipid II at a different site than vancomycin. Furthermore, unlike daptomycin, ambocidin A retains potent antimicrobial activity in the presence of lung surfactant, giving it the potential to treat bacterial pneumonia. Our work expands the family of calcium-dependent lipopeptide antibiotics with a new member exhibiting a distinct mechanism of action.
Introduction
Calcium-dependent cyclic lipopeptides, exemplified by the FDA-approved daptomycin, are potent Gram-positive active antibiotics. Cyclic lipopeptides are produced by bacteria using modular non-ribosomal peptide synthetase enzymes that catalyse the condensation of both proteinogenic and non-proteinogenic amino acids, along with at least one fatty acid, followed by cyclisation.1 In the clinic, daptomycin is used for the treatment of complicated skin and structure infections caused by methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecium (VRE),2 as well as MRSA endocarditis and bacteriaemia.3 Despite these successes, the development of resistance to daptomycin is documented by several mechanisms.1 Especially concerning is the cross-resistance between daptomycin and the last resort glycopeptide antibiotic vancomycin.4 Daptomycin is also unable to treat infections of the lung as its effectiveness is severely attenuated by pulmonary surfactant.1 While Ca2+-dependent cyclic lipodepsipeptides are united in the binding of calcium, which is hypothesised to neutralise charge on the peptide to enable interaction with the cell wall or membrane,5 they often have distinct mechanisms of action from one another.6 There is therefore a clear incentive for finding and characterising more examples of calcium-dependent cyclic lipodepsipeptides with enhanced bioactivity, ability to overcome resistance mechanisms, or bioavailability.7
The methods used to find new bioactive natural products have changed greatly since the 1970s. Where bioactivity-based screening (the Waksman method) was once prolific in finding new antibiotics, today a less direct approach is often required to avoid reisolating known compounds. The availability of vast databases of microbial genome sequence data has facilitated the identification of hundreds of thousands of gene clusters encoding the enzymes responsible for natural product biosynthesis (called biosynthetic gene clusters or BGCs).7a, 8 This abundance of microbial genome-sequence data has enabled several key insights: the first being that in a laboratory setting, microbes typically only produce a fraction of the natural products encoded in their genomes. Most biosynthetic gene clusters are transcriptionally silent (possibly from the lack of precise environmental cues that trigger activation).9 Obtaining access to this vast pool of latent natural products is an opportunity to explore new regions of chemical space for next-generation antibiotics.
Another key development arising from the proliferation of microbial genome sequence data has been the development of robust, automated tools for identification,10 annotation and comparison7b, 7c of natural product biosynthetic gene clusters (BGCs). Widespread application of these tools has revealed that for many FDA-approved natural product drugs, silent BGCs encoding potentially related compounds are abundant in existing strain repositories.7 Once identified, such BGCs may be cloned and transferred to a heterologous bacterial host capable of expressing genes that were dormant in the native host, leading to the production of a previously “invisible” natural products.11 Heterologous expression of BGCs been a particularly fruitful method for the discovery of new Ca2+-dependent lipopeptides.11b, 11d, 12
Here we describe the use of genome mining, whole-BGC cloning, and heterologous expression in multiple Streptomyces hosts to discover the ambocidins: cyclic lipodepsipeptides whose BGC is found in the genome of the heavily studied bacterial species Streptomyces ambofaciens. The ambocidins have potent bioactivity against numerous antibiotic resistant Gram-positive human pathogens. Unlike daptomycin, the ambocidins are not inhibited by serum or pulmonary surfactant, broadening their potential application. The ambocidins target lipid II in the bacterial cell wall, however they bind a different region than vancomycin, enabling them to kill vancomycin-resistant pathogens. The rare amino acid Nϵ-hydroxyarginine in the ambocidins is important for bioactivity, leading us to identify the hydroxylase enzyme (AmbT) required for its biosynthesis. Using the AmbT protein as a query we were able to find BGCs encoding large non-ribosomal peptides that might serve as fruitful targets for future genome mining efforts.
Results and Discussion
Analysis of the Ambocidin Biosynthetic Gene Cluster
A recent genomic survey of actinomyces species for BGCs encoding calcium-dependent antibiotics uncovered multiple species that had the genetic capacity to produce previously undescribed compounds.13 Among these, a silent BGC found in the genome of Streptomyces ambofaciens ATCC 23877 was of particular interest as the order and predicted substrate specificity of adenylation domains in the BGC indicated that the product was likely to be broadly different from anything previously described.13 We conducted our own analysis of the genome of S. ambofaciens ATCC23877 using antiSMASH 6.014 and identified a 75 kb BGC that contains the biosynthetic features consistent with the production of a calcium-dependent lipopeptide (Figure 1A, Supporting Information Table S7). Manual examination of the genes at this locus (which we named the amb BGC) identified at least 23 putative biosynthetic genes (ambA–W). The amb cluster contains three nonribosomal peptide synthetase (NRPS) genes, ambD, ambI, and ambS, with a total of 13 predicted modules–indicating a 13-residue non-ribosomal peptide. In addition to the core NRPS biosynthetic genes, the amb BGC contains genes for biosynthesis of non-canonical amino acids, as well as genes for fatty acid biosynthesis and attachment.
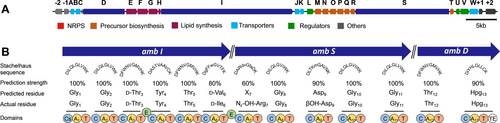
The ambocidin biosynthetic gene cluster. (A) Colour coded scale map of the entire amb BGC. (B) Core NRPS genes with details of module arrangement, substrate binding pocket, substrate specificity predictions and actual activated substrates.
Discovery of Ambocidin Cyclic Lipodepsipeptides via Heterologous Expression
Despite extensive characterisation of S. ambofaciens ATCC 23877 secondary metabolites,15 no calcium-dependent antibiotic activity has ever been reported, suggesting the amb BGC is not expressed under commonly employed laboratory cultivation conditions. We cultivated S. ambofaciens using six media commonly found to elicit antibiotic production16 and did not observe any calcium-dependent antibiotic activity, indicating that the amb cluster is likely to be transcriptionally silent or non-functional in its native host (Supplementary Figure S34).
As transcriptionally silent gene clusters arise from intrinsic and often cryptic repression mechanisms in the native host,17 we sought to move the entire amb cluster into a more permissive heterologous host. Our initial attempts to clone the amb cluster using CRISPR-Cas9 targeted transformation-assisted recombination (TAR) in yeast were unsuccessful, as can be the case with large biosynthetic gene clusters. We then turned to the recently developed CAPTURE method of whole-BGC cloning, which uses Cas12a to excise the BGC of interest followed by in vitro assembly with two DNA adaptors using the 3’–5’ exonuclease activity of T7 DNA polymerase.11a, 18 The resulting linear product is transformed into E. coli cells expressing Cre recombinase, which catalyses the recombination between the LoxP site on the free ends of each DNA adaptor–forming a circular plasmid.11a, 18 The original protocol uses LacZ/β-galactosidase screening to identify successful Cre recombination events. To streamline the protocol we replaced the lacZ gene with pheS, the product of which catalyses the incorporation of the 4-chorophenylananine (4-CP) into proteins, resulting in misfolding and cell death. Since pheS is removed following successful Cre-catalysed circularisation, the supplementation of 4-CP in the growth medium selects for correctly assembled BGC-containing plasmids. The use of 4-CP is additionally advantageous for being far cheaper than the β-galactosidase required for LacZ blue/white screening.
Using our optimised CAPTURE method we cloned the complete amb BGC and verified the final construct by PCR and restriction enzyme digestion of the purified plasmid (Supplementary Figure S1). We then conjugated the amb cluster into four Streptomyces strains commonly used for the heterologous expression of BGCs: Streptomyces avermitilis SUKA 17,11a, 19 Streptomyces albus Del14,20 Streptomyces coelicolor M115221 and Streptomyces venezuelae ATCC 15439.22 Exconjugants were fermented for 10 days, followed by solid phase extraction of the culture supernatants. Comparison of HPLC traces of each amb cluster-containing heterologous host to its corresponding empty-vector control revealed the production of new metabolites in S. avermitilis SUKA 17::amb, S. albus Del14::amb, and S. coelicolor M1152::amb, but not S. venezuelae::amb (Figure 2A.).
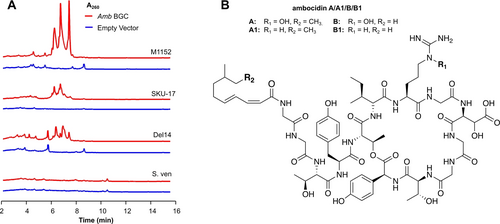
Heterologous expression of the ambocidin biosynthetic gene cluster. (A) HPLC traces for four heterologous host strains comparing the presence of the amb BGC to an empty vector control (B) Elucidated structures of the biologically active ambocidins isolated via heterologous expression. M1152=S. coelicolor M1152, S. ven=S. venezuelae ATCC 15439, Del14=S. albus Del14, SUKA 17=S. avermitilis SUKA 17.
In all three hosts where new peaks were apparent in HPLC traces, new [M+H]+ ions 947.5197 and 933.5038 were also observed, and these ions co-eluted with their putative dehydroxylated congeners 931.5248, and 917.5091 (Supplementary Figure S4). Surprisingly, only the organic extract of S. avermitilis SUKA-17::amb exhibited calcium-dependent antibiotic activity against Bacillus subtilis (Supplementary Figure S6). Manual comparison of the organic extracts of the three producing heterologous hosts by HPLC-MS revealed the additional ions [M+H]+1481.6907 (ambocidin A) and 1467.6749 (ambocidin B)–along with the respective dehydroxylated congeners 1465.6958 (ambocidin A1) and 1451.6801 (ambocidin B1)–exclusively present in S. avermitilis SUKA-17::amb (Figure 3A, Supplementary Figure S4).
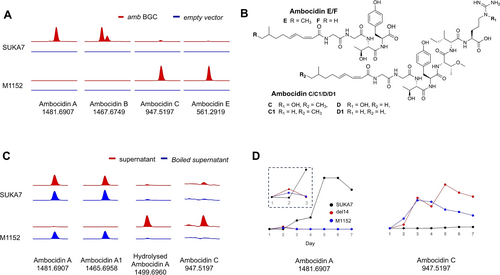
Heterologous host dependent production of truncated ambocidins: (A) LC–MS analysis of crude extracts of two heterologous Streptomyces hosts harbouring the amb BGC and empty vector negative control. (B) Assigned structures of truncated ambocidins C−F based on MS2 fragmentation analysis. MS/MS data is provided in Supplementary Figure S2. (C) Treatment of Ambocidin A/A1 with concentrated culture supernatant from the three heterologous hosts used in this study. EIC of each ambocidin compound was extracted at indicated m/z values with 20 ppm window. (D) Time course sampling of ambocidin production in different heterologous hosts. Data for ambocidin A, and C are shown. Data for the remaining ambocidins are presented in Supplementary Figure S9.
We hypothesised the larger ions we observed (ambocidins A/A1 and B/B1) correspond to the mature, bioactive product of the BGC, while the smaller ions (ambocidins C−F) detected in S. albus Del14 and S. coelicolor M1152 are degradation products. In support of this hypothesis, we found that ambocidin A is actually detectable in S. albus Del14::amb and S. coelicolor M1152::amb, but only up to three days post culture inoculation, after which it is no longer present (Figure 3D, Supplementary Figure S10). To determine if this degradation is enzymatic, we incubated purified ambocidin A/A1 with concentrated supernatants or cell lysates from S. albus Del14, S. coelicolor M1152 and S. avermitilis SUKA1 cultures. Only S. albus Del14 or S. coelicolor M1152 culture supernatants were able to degrade ambocidin A/A1 to ambocidin C/C1 (Figure 3C, Supplementary Figure S7). Boiling the culture supernatant (Figure 3C, Supplementary Figure S7) or adding a broad spectrum metalloprotease cocktail (Supplementary Figure S8), prevented ambocidin A/A1 degradation, clearly implicating a protease enzyme that was either excreted, or released due to cell lysis.
We isolated ambocidin A/A1/B/B1 from S. avermitilis SUKA-17::amb using calcium-induced precipitation and reverse phase chromatography. The amino acid composition and connectivity for each metabolite was elucidated by NMR spectroscopy and LC–MS/MS (Supplementary Methods S12, Supplementary Figure S2, Supplementary Figures S13–S33, Supporting Information Tables S2–S3). With the exception of β-carbon of the β-hydroxy aspartic acid residue and the penultimate carbon in the lipid substituent, stereochemistry of all chiral centres was determined by comparison to reference standards using C3-Marfey's analysis23 (Supplementary Figure S5, Supporting Information Table S4). Ambocidin A/A1/B/B1 are cyclic lipodepsipeptides comprised of 13 amino acids with a central 28-membered non-homodetic ring (Figure 2B). Ambocidin A and B contain the rare amino acid l-Nϵ-hydroxy-arginine at position seven, while ambocidin A1 and B1 simply have unmodified l-arginine. The ambocidins are lipidated via the amino group of their N-terminal glycine residue. In the case of ambocidin A and A1, the attached fatty acid is (2Z,4E)-8-methyldeca-2,4-dienoic acid. The fatty acid attached to ambocidin B and B1, (2Z,4E)-8-methylnona-2,4-dienoic acid, is one carbon shorter.
Having accurate masses for the products of the amb BGC allowed us to specifically search LC–MS/MS chromatograms of extracts from S. ambofaciens for evidence of ambocidin production. This analysis confirmed that production of ambocidins A–F is repressed in S. ambofaciens. Interestingly, integration of a second copy of the entire amb BGC into the chromosome of S. ambofaciens appears to partially bypass repression, leading to production of ambocidin A, albeit at levels approximately 40 fold lower than seen with heterologous expression (Supplementary Figure S35).
Bioactivity and Structure-Activity Relationship
Ambocidin A is active against the Gram-positive bacteria Bacillus subtilis, Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Enterococcus faecium (VRE). The minimal inhibitory concentration (MIC) values for ambocidin A range from <0.031 to 1 μg/mL in broth dilution assays (Table 1). In contrast, ambocidin A1 exhibits a 8–16 fold increase in MIC against all tested Gram-positive bacteria except B. subtilis, clearly implicating the Nϵ−OH group in Arg7 as a key feature for bioactivity. Ambocidin B has similar MIC values to ambocidin A1, while ambocidin B1 has greatly reduced potency compared to ambocidin A (>64 times less active against S. aureus and MRSA) Taken together, these data indicate that both fatty acid length and the hydroxylation state of Arg7 are important factors for ambocidin bioactivity, with ambocidin A being the most potent congener. None of the ambocidins are active against any of the Gram-negative bacteria tested, nor did any exhibit toxicity against HCT-116 human colon carcinoma cells (MIC>32 μg/mL). Ambocidin A was assessed for haemolytic activity at concentrations up to 200 μg/mL, and did not result in any detectable haemolysis (Supplementary Figure S36). The activity of ambocidin A was found to be calcium dependent when tested against a panel of Gram-positive pathogens at a range of calcium concentrations, with higher concentrations of calcium resulting in lower MIC values against all strains tested (Figure 4A, Supporting Information Table S9)
|
B. subtilis |
S. aureus |
MRSA |
VRE |
E. coli |
K. pneu. |
A. baum. |
HCT-116 |
|---|---|---|---|---|---|---|---|---|
Ambocidin A |
<0.031 |
0.25–0.5 |
0.5–1.0 |
0.25 |
>50 |
>50 |
>50 |
>32 |
Ambocidin A1 |
<0.031 |
4.0 |
4.0–8.0 |
4.0 |
>50 |
>50 |
>50 |
>32 |
Ambocidin B |
<0.031 |
4.0 |
4.0 |
2.0 |
>50 |
>50 |
>50 |
>32 |
Ambocidin B1 |
1.0 |
>32 |
>32 |
32 |
>50 |
>50 |
>50 |
>32 |
Daptomycin |
0.125 |
<0.01 |
0.031 |
0.125 |
>50 |
>50 |
>50 |
ND |
Vancomycin |
0.25 |
2.0 |
1.0 |
>32 |
>50 |
>50 |
>50 |
ND |
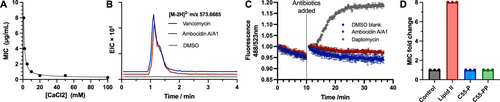
Mechanism of action of the ambocidins: . (A) Minimal inhibitory concentration (MIC) assays of Ambocidin A against S. aureus at different CaCl2 concentrations. Identical MIC values for each concentration were obtained from three biological replicates. (B) HPLC-HRMS analysis of cell lysate of MRSA USA 300 incubated with either ambocidins A/A1, DMSO (negative control) or vancomycin (positive control), monitoring the [M-2H]2- ion of UDP-MurNAc-pentapeptide (m/z=573.6685) in ESI negative mode. (C) Membrane lysis assay with SYTOX Green dye against MRSA USA-300 incubated with either ambocidins A/A1, daptomycin (positive control) or DMSO (negative control). Data is plotted as average of three technical replicates +/- standard deviation and is representative of replicate experiments performed on two separate days. (D) MIC fold change of ambocidins A/A1 against B. subtilis in the presence of three different membrane lipid components: Lipid II purified from S. aureus, undecaprenyl phosphate (C55-P) and undecaprenyl pyrophosphate (C55-PP), added at 5 : 1 molar ratio to ambocidins A/A1. A solvent blank control is included as negative control. Data is plotted as average of three independent experiments performed on separate days with individual data points shown as solid circles.
Determination of the Mechanism of Action of the Ambocidins
Cyclic lipopeptide antibiotics typically target components of the Gram-positive bacteria membrane such as lipid II,11b undecaprenyl monophosphate (C55-P),24 or undecaprenyl diphosphate (C55-PP).24a To determine the mechanism of action of the ambocidins, we used HPLC-MS to monitor metabolic changes in S. aureus upon exposure. We observed the accumulation UDP-MurNAc-pentapeptide with ambocidin A treatment, indicating inhibition of cell wall biosynthesis (Figure 4A). Next we monitored membrane disruption by using SYTOX green dye and found that, unlike daptomycin, ambocidin A/A1 does not disrupt the MRSA cell membrane (Figure 4B). To determine which cell wall component ambocidin A targets, we supplemented purified lipid II, C55-P or C55-PP, into B. subtilis cultures, followed by challenging with ambocidin A/A1. The addition of lipid II at 5 : 1 molar ratio completely rescued B. subtilis from ambocidin-induced antibiosis. In contrast, C55-P and C55-PP at similar molar ratios had no effect on antibiosis (Figure 4D). The clinically used antibiotic vancomycin also targets lipid II, specifically binding to its d-ala-d-ala motif. Vancomycin-resistant Enterococcus faecium (VRE) is altered at this motif through a missense mutation, significantly attenuating vancomycin affinity. That the ambocidins all have potent activity against VRE indicates that they use a inhibition mechanism that does not bind the d-ala-d-ala. Furthermore, we found that a 200-fold molar excess of diacetyl-lys-d-ala-d-ala-OH (which mimics the d-ala- d-ala motif on lipid II) has no effect on ambocidin A bioactivity, whereas vancomycin is antagonised by a 12-fold molar excess (Supporting Information Table S5), providing further evidence that the ambocidins bind lipid II at a different location from that of Vancomycin. Taken together, our experiments demonstrate that the ambocidins inhibit cell wall biosynthesis–without causing membrane disruption–by antagonising lipid II at a site other than the well-established d-Ala-d-Ala motif targeted by vancomycin.
The effectiveness of the clinically used lipopeptide antibiotic daptomycin is blunted in the presence of serum or pulmonary surfactant.25 To determine if ambocidin A is similarly affected, we measured the MIC of ambocidin A against B. subtilis in the presence of each of these. The MIC only increased two-fold in the presence of 10 % human serum, and four-fold with 1 % pulmonary surfactant. In contrast, the daptomycin MIC increases 8-fold when incubated with 10 % human serum and more than 256-fold increase with 1 % pulmonary surfactant (Supporting Information Table S6). Ambocidin A is therefore only mildly affected by these clinically relevant physiological factors.
Assignment of AmbT as an Arginine Nϵ-Hydroxylase
To understand the biosynthetic origin of the important bioactivity-enhancing Nϵ-hydroxyarginine in the ambocidins, we performed a targeted gene deletion. Among the genes present in the amb gene cluster, we identified ambT as the most likely candidate for catalysing arginine hydroxylation. The AmbT protein was identified by the built-in pHMM scan functionality of antiSMASH 6.0 as belonging to the YqcI/YcgG family of heme-dependent oxygenases (e-value for hit to PF08892=9.1e–57), members of which have been shown to catalyse Nϵ-hydroxylation of arginine in the biosynthesis of miharamycin26 and argolaphos27 (Figure 5A). To confirm the role of ambT in arginine hydroxylation we used a modified form of CAPTURE to disrupt ambT with a kanamycin-resistance cassette in-vitro, creating amb::ΔambT. Vexingly, despite repeated attempts to conjugate ambΔambT into S. avermitilis we were unable to obtain any exconjugants. As a workaround, we delivered amb::ΔambT into S. coelicolor M1152, which yielded viable exconjugants. HPLC-HRMS analysis of the resulting fermentation extracts showed that production of Nϵ-hydroxyarginine-containing ambocidins (ambocidins C/D) was completely abolished and that the non-hydroxylated congeners (ambocidins C1/D1) are the new major products (Figure 5B, Supplementary Figure S9). The abolished production of Nϵ-hydroxyarginine in the hosts containing amb::ΔambT clearly demonstrates that AmbT is the enzyme responsible for catalysing this important transformation in ambocidin biosynthesis.
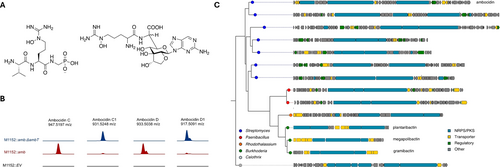
Confirmation of AmbT as an arginine Nϵ-hydroxylase and in-silico screening for other non-ribosomal peptide BGCs containing this gene . (A) Structures for argolaphos (left) and miharamycin A (right). Previously characterised examples of arginine Nϵ-hydroxylation catalysed by ambT like proteins belonging to the YqcI/YcgG family of heme-dependent oxygenases. (B) Extracted ion chromatograms from HPLC-HRMS analysis comparing cultures of S. coelicolor M1152 harbouring the complete amb gene cluster (M1152::amb) to M1152 harbouring amb BGC lacking the ambT gene (M1152::amb:ΔambT) to) and an empty vector control. Extracted mass chromatograms for the hydroxylated products (ambocidins C and D) and corresponding dehdroxylated congeners (ambocidins C1 and D1) are presented. (C) Comparison of the ambocidin BGC to other BGCs putatively encoding large non-ribosomal peptides with one or more Nϵ-hydroxyarginine moiety. The phylogenetic tree was constructed using an alignment of the AmbT-like proteins present in each BGC. Further details for each BGC in this Figure are given in Supplementary table S8.
YqcI/YcgG Family Arginine Nϵ-Hydroxylases Markers for Structural Novelty and Bioactivity
Nϵ-hydroxyarginine is a rare structural motif in microbial natural products. By searching the Dictionary of Natural products for this moiety, we were able to identify just four compound series’: the miharamycins,26 asterobactins,28 pentaminomycins29 and argolaphos.27 Addition of the ambocidins to this group brings the total number of reported instances of this motif to five, with three of these possessing antimicrobial activity. This result suggested to us that proteins in the YqcI/YcgG family might serve as a useful biosynthetic marker for both structural novelty, and clinically relevant bioactivity. To investigate this possibility further, we conducted a search of all sequenced bacterial genomes available in NCBI for BGCs encoding large non ribosomal peptides (>=8 Adenylation Domains) and at least one ambT homologue using the Boolean search functionality of the antiSMASH database. This search returned 124 BGCs, the majority of which were similar or identical to the BGCs siderophores plantaribactin, gramibactin and megapolibactin. Each of these BGCs encode compounds that contain the amino acid graminine, a moiety which is derived from arginine via an as yet unknown biochemical transformation.30 By further refining our search to exclude siderophores, followed by manual removal of BGCs that appeared to be identical or near-identical to those for previously described compounds, we were left with ten unique BGCs (Figure 5C, Supporting Information Table S8). Among these ten BGCs, one appeared to encode a compound that was very similar, or perhaps identical, to the ambocidins (Supplementary Figure S12) and four further BGCs had a calcium dependent antibiotic as their closest match among all characterised BGCs in the MiBiG database (Supporting Information Table S8). We posit that the BGCs identified in this analysis, particularly those whose closest characterised relative encodes an antibiotic, might serve as a fruitful targets for future genome mining efforts.
Conclusion
The ambocidins are calcium-dependent antimicrobial cyclic lipopeptides that are active against Gram-positive bacteria. The ambocidins target Lipid II and have a binding site that differs from that of vancomycin. The discovery of the ambocidins was facilitated by the use of multiple heterologous hosts for expression of the amb BGC, only one of which produced new biologically active cyclic lipopeptides. The choice of heterologous host was therefore important not only for binary presence/absence of products, but also for the isolation of active compounds that would have been hidden if an appropriate host had not been chosen for expression. By assessing the biological activity of four cyclic ambocidin congeners, we were able to determine that both the Nϵ−OH group on arginine7, and the length of the attached lipid chain had a strong influence on potency. Future synthetic or semi-synthetic efforts might focus on varying these moieties for the generation of improved variants. The ambocidins are previously uncharacterised antimicrobials with clinically relevant biological activity. Their discovery from a BGC that was dormant in an extensively studied Streptomyces strain highlights the utility of heterologous expression in the ongoing race to discover new lead compounds to combat the rise of drug-resistant bacterial infections.
Contributions
HEL and JGO conceived the project. HEL, JGO and RFL and wrote the manuscript. HEL performed all experiments except where credited as follows: VHW and EFW performed NMR experiments; VHW performed mammalian cytotoxicity assay; JGO and RFL isolated compounds; EFW performed Marfey's analysis; JGO, VHW and EFW elucidated structures. JGO obtained funding. JGO and RAK supervised the project.
Acknowledgments
We would like to thank Drs. Ewa Swiezewska and Karolina Skorupinska-Tudek from Institute of Biochemistry and Biophysics, Polish Academy of Sciences (IBB PAS) for their gift of undecaprenyl monophosphate (C55P). Open Access publishing facilitated by Victoria University of Wellington, as part of the Wiley - Victoria University of Wellington agreement via the Council of Australian University Librarians.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data except NMR data files are available in the Supporting Information. NMR data files (FID) have been deposited in Zenodo. The DOI for NMR data files deposited in Zenodo is: 10.5281/zenodo.13905661




