An Atomic-Scale Explanation for The High Selectivity Towards Carbon Dioxide Reduction Observed On Liquid Metal Catalysts
Abstract
The low-temperature liquid metals Ga-In and Ga-Sn have previously showcased >95 % selectivity towards the electrochemical reduction of CO2 to formate, occuring only when the alloys are melted, not solid. Here, density functional theory molecular dynamics and metadynamics simulations reveal that CO2 does not directly adsorb to the Ga-alloy surface, but instead is reduced indirectly by reaction with an adsorbed hydrogen. The reaction barrier is vastly more favourable when this process occurs at In or Sn sites (average: 0.26 eV), than when it occurs on Ga (average: 0.47 eV). However, there is no difference in barrier between solid and liquid surfaces. Instead, we find that Hads is mobile only on the liquid surface, travelling due to the motion of the liquid beneath. This process drives Hads to In/Sn sites, allowing low-barrier CO2 reduction to occur only on the liquid. Therefore, the dynamic motion of liquid metal catalysts can underpin their unique reactivity. The result has far reaching implications for any protonation reaction conducted with a liquid metal catalyst.
Liquid metal catalysts have received growing recognition as their unique reactivity begins to emerge.1-4 These fascinating materials are so named due to their low melting points (<330 °C),5 yet they maintain high conductivity and metallic properties in liquid form.3, 6 As catalysts, liquid metals have been known to offer impressively high activities, sometimes outcompeting their solid counterparts by orders of magnitude.2, 7, 8 Their dynamic character can make them resistant to processes which would degrade traditional solid catalysts over time (e.g. coking),2, 9 and many liquid metals (often Ga-based) remain stable over wide temperature ranges.6 Despite their disordered nature, there are a number of examples where liquid metal catalysts are able to offer high reaction selectivity towards specific products.2, 10, 11 In some cases, this selectivity has been suggested to be a result of the surface element types available on a liquid metal surface,12 and in other cases the dynamic nature of the liquid metal itself is believed to be critical.13
A very important example of reaction selectivity in liquid metals was reported in 2021 by Liu et al.,12 where Ga-In and Ga-Sn alloys showcased 95 % selectivity towards electrochemical CO2 reduction to formate in their liquid form, but could drive only hydrogen evolution when solid. The authors attributed the remarkable selectivity on melting to the migration of dopant (In or Sn) from the bulk of Ga to the surface. It was suggested that new surface In and Sn offer active sites for CO2 reduction, thus switching the activity from hydrogen evolution to formate production. While atomic-scale modelling has supported that this dopant migration does occur on melting,14 we suggest there must still be additional factors in controlling the reaction selectivity that remain to be elucidated. The reasons for this are two-fold: (1) If reaction selectivity is purely due to CO2 reduction strongly outcompeting hydrogen evolution at dopant sites, this does not explain why CO2 reduction is barely observed (<10 % Faradaic efficiency) in the solid, which has surface dopant concentrations around 6.3–6.6 atomic (at.) %.12 The melting of the Ga-alloys can also be cycled, where solidification may leave even greater quantities of dopant at the surface. Yet, when re-solidified, the reaction selectivity to CO2 reduction is still observed to switch off.12 Furthermore, if one assumes all the dopant in a liquid Ga-alloy with 8.2 at. % In/Sn migrates to the surface on melting, this still leaves approximately 75 % of the surface atoms as Ga.14 To produce the 95 % selectivity towards formate observed in experiment, these dopant sites would have to be significantly favoured over Ga, which is inconsistent with the lack of CO2 reduction on the solid system. (2) Very recent work has suggested that it is specifically the dynamic motion of atoms on the surface of liquid metal catalysts that enable certain reactivity.13, 15 For instance, the dynamic nature of a liquid GaSn0.029Ni0.023 catalyst has been found to allow configurational matches with reactants (occurring on a statistical basis over time) that let reaction pathways proceed.13 Applied to alkane cracking reactions, the existence of certain surface atoms (e.g. Ni, Sn) may be a prerequisite for catalysis, yet the authors suggested it was the dynamic flexibility of the liquid that conferred selectivity towards propane. In this way, the dynamics of a liquid catalyst could also be the factor that enables the CO2 reduction pathway on Ga-In and Ga-Sn. Taken together, these factors lead us to suspect there must be a pronounced change in character when the Ga-alloys are melted that enables appreciable CO2 reduction, perhaps in addition to the moderate increase in accumulation of surface dopant.
In this communication, we report an atomic-scale understanding of the dynamic surfaces of Ga-In and Ga-Sn that allows us to specifically demonstrate how CO2 reduction is favourable on the liquid, but less likely on the solid. To achieve this we employ a recently-developed dynamic sampling technique to calculate the energy of surface bound adsorbates, which is explained in detail in Ref. [15]. Following this, the key barriers to CO2 reduction were determined using metadynamics simulations.16-18 It is critical to employ a transition state search method that allows the catalyst to remain as an “at-temperature” dynamic liquid to capture any arising differences from the solid. Additionally, there is literature precedent for metadynamics correctly modelling the reduction of small carbonaceous species.19
The Ga-alloy systems were modelled using density functional theory (DFT) with a Perdew–Burke–Ernzerhof for solids (PBEsol) functional20 as implemented in the Vienna Ab initio Simulation Package (VASP).21 Starting from a 192-atom hexa-layer, which has been generated via simulated annealing of a cut of bulk α-Ga and equilibrated at temperature (290 K for solid, 450 K for liquid) for 150 ps,14, 15 a smaller 72-atom model was cut while maintaining the 6-layer structure of the Ga. The dopant (In or Sn) was initially added randomly at 8.3 at. %, and the structures were equilibrated at temperature for at least 40 ps via DFT molecular dynamics (1 fs timestep). It should be noted that the melting temperatures reported for these slab models of Ga-In and Ga-Sn deviate from those of the bulk alloys. This is consistent with past work finding that finite slabs of Ga materials melt at higher-than-bulk temperatures.22 Additional details on the methodology are reported in the Supporting Information.
Dynamic sampling simulations of CO2 on Ga-In and Ga-Sn showed that the small molecule was unable to adsorb at either Ga or dopant sites, and would rapidly leave the liquid surface. Indeed, this pattern was also observed for fully relaxed surfaces of the Ga-alloys, where CO2 would also leave the surface during the course of an optimisation. Previous literature on solid surfaces of certain post-transition metals (e.g. Sn and Pb) has indicated direct adsorption and reduction of CO2 is unlikely and would come at a high energy cost.23, 24 Instead, CO2 may be indirectly reduced by surface-adsorbed hydrogen.25 The involvement of Hads in CO2 adsorption is also observed for Cu nanoparticle catalysts, without which there is only a weak association between adsorbate and catalyst.26, 27 Consistent with this idea, we find that the adsorption of CO2 on liquid Ga-In and Ga-Sn occurs in a hydrogen-assisted manner, where a surface-bound H reacts with CO2 as it adsorbs to make formate. CO2 reduction therefore requires that a H+ from solution be first reduced and adsorbed, and that the CO2 and resulting Hads come into close proximity.
We have previously conducted dynamic adsorption sampling of the Hads state on liquid Ga-In and Ga-Sn,15, 28 yielding average adsorption energies of 0.26 and 0.27 eV, respectively. This is slightly unfavourable relative to the H2 reference state. In contrast, the surface-adsorbed formate that is produced by indirect CO2 reduction is steeply downhill in energy,15 with an average adsorption energy of −0.99 eV on Ga-In and −0.80 eV on Ga-Sn. A full record of these adsorption energies, including those for the solid systems, is presented in the Supporting Information.
A series of metadynamics simulations were conducted to probe this hydrogen-assisted adsorption pathway on the liquid Ga-alloy catalysts. The distance between the C in CO2 and the surface H (dC−H) was selected as the relevant collective variable to sample along. The resulting transition states (Figure 1) indicated that CO2 would descend to the surface near the adsorbed H, then would arrange into a bent configuration with the carbon pointing at Hads and usually one oxygen pointing towards the surface. At this point, CO2 could be protonated and the subsequently formed formate would rotate to point both oxygen atoms towards surface Ga in the previously identified most stable binding configuration.15
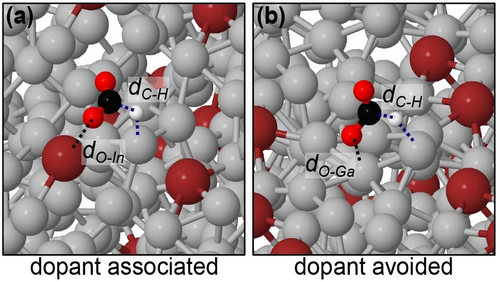
Transition states for proton-assisted CO2 reduction on liquid Ga-In when (a) CO2 is allowed to react at the preferred site involving In, and (b) when CO2 is biased away from In and must react at a Ga. The dashed black line indicates the coordination of oxygen to a surface atom so that CO2 may form a bent configuration. The blue lines indicate the breaking surface-H bonds and the newly forming C−H bonds.
In initiating several metadynamics pathways from different starting configurations on Ga-In, a common theme was observed: the resulting transition state tended to involve a surface dopant atom (i.e. In). The geometry of the transition state was characterised by a CO2 coordinating a single oxygen atom to the surface dopant to form a bent configuration, while acquiring the Hads from a neighbouring Ga. A representative example of this transition state is depicted in Figure 1a. Although there was some statistical variance with respect to how rapidly a given metadynamics simulation located a transition state with this character, it eventually occurred in all the free pathways that were tested with an average energy barrier of eV (95 % confidence interval). Very similar transition states were also repeatedly located on liquid Ga-Sn surfaces, where CO2 would coordinate to surface Sn as it did for In. Therefore, we broadly group this type of pathway as “dopant associated”.
To more directly show the favourability of CO2 reduction occurring at a dopant site, separate metadynamics simulations were conducted for both Ga-In and Ga-Sn under two different restrictions: (a) One of the O atoms in CO2 was affected by a sigmoidal bias potential which pushed it in the direction of a surface dopant atom if it ever exceeded 4.5 Å in distance. This produced transition states like the dopant associated one shown in Figure 1a. Alternatively, (b) the C atom in CO2 was biased away from approaching within 5 Å of a surface dopant, forcing the reduction reaction to occur on Ga (i.e. “dopant avoided”, characteristic transition state shown in Figure 1b). Four metadynamics simulations were conducted under each restriction, and for both solid and liquid structures of each Ga-alloy. The average activation barrier, and the 95 % confidence interval estimate on this value are shown in Figure 2. The dopant associated CO2 reduction barriers are markedly more favourable, sitting at approximately half the magnitude of those where dopant is avoided. Furthermore, there is no difference between the magnitude of these barriers on Ga-In versus Ga-Sn, reinforcing the existing literature that suggests their reactivity is alike.12, 28 These findings are highly consistent with Liu et al.’s12 hypothesis that surface In and Sn are required for selective CO2 reduction to proceed. However, when analogous metadynamics simulations are conducted on solid Ga-In and Ga-Sn, near-identical barriers and behaviour are also observed. As for the liquid, the solid alloys have dopant associated barriers that are approximately half as high compared to when the CO2 to formate reaction happens at Ga.
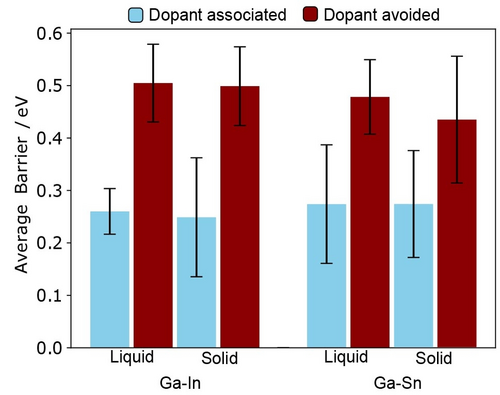
Activation barriers for proton-assisted CO2 reduction on solid and liquid surfaces of Ga-In and Ga-Sn. The blue bars show the lower barrier process where dopant is allowed to coordinate to CO2 to assist the transfer of H. The red bars show the barrier in cases where this coordination is prohibited, and the reaction happens at a Ga instead. The 95 % confidence interval from metadynamics replicates is shown on the plot.
Interestingly, the marked similarity in the barriers between solid and liquid surfaces implies that the CO2 to formate pathway would also dominate over hydrogen evolution on the solid Ga-alloys, given that an appreciable amount of surface dopant atoms are available as active sites. Recall that Liu et al. experimentally measured between 6.3 and 6.6 at. % concentrations of surface dopant in solid Ga-In and Ga-Sn, respectively.12 While these concentrations were enriched up to 10.5 at. % on melting, the surface dopant in the solids still offers ample sites for CO2 reduction, especially given the very low activation barriers (around 0.25 eV). Yet, because Liu et al. observed only around 10 % Faridaic efficiency to formate in these solid systems,12 we propose that there must be an additional change to the surface on melting that leads to the high formate selectivity, on top of the additional dopant sites being made available.
We return to the previously proposed idea that the dynamic motion of a liquid metal surface is important to its catalytic activity.13, 15 Because CO2 adsorbs to the surface through a proton assisted pathway, and this favourably happens at a dopant site, we argue that a key step in the process must also be the diffusion of Hads along the surface to reach a site that neighbours Sn or In. We have recently studied the adsorption of H on Ga-In and Ga-Sn, finding that it favours adsorbing at Ga sites.28 Critically, we also observed that hydrogen tended to have low mobility on the solid surfaces, as diffusion involved traversing regions where H has not bound favourably. In contrast, on the liquid surface, the constant rearrangement of atoms instead provided pathways for hydrogen to diffuse. At times, the Hads was actually forced to move on the surface as the atom it was bound to rearranged passively at temperature. In this way, hydrogen is actually driven across the liquid surfaces, almost akin to surfing over the motion of the dynamic liquid.
To examine this effect more quantitatively here, we considered solid and liquid systems with one surface dopant atom, and placed an adsorbed hydrogen at a distance of approximately 7 Å away. The systems had previously been equilibrated at 290 K and 450 K, respectively. The H-dopant distance on the surface was tracked over a 25 ps simulation time (shown for Ga-In in Figure 3), in order to gauge the likelihood of H being brought close enough to the dopant site to react. Based on the dopant associated pathways calculated with metadynamics, we estimate that H would have to be within approximately 4 Å of the surface dopant, and situated on a neighbouring Ga to react in this manner.
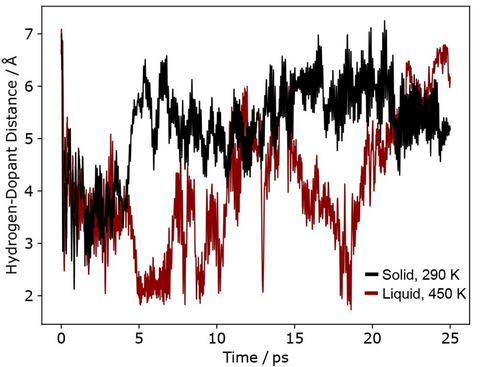
The distance between Hads and a single surface In atom tracked over a 25 ps ab initio molecular dynamics run on solid and liquid Ga-In. In metadynamics simulations, all dopant associated reaction events occur with H situated within 4 Å of the dopant atom. H in the liquid system regularly approaches within this distance, which is not the case on the solid.
Figure 3 shows that, for both solid and liquid, Hads initially has high mobility on the surface. This is a natural effect of placing Hads onto an already equilibrated surface at temperature, at a binding site which may not be very stable. After 5 ps into the simulation, the Hads is able to find preferred adsorption sites on the Ga atoms. For the solid, Hads then remains associated with this binding site for the remaining 20 ps of simulation time, with its distance to In at a mean of 5.5 Å and varying with a range of approximately 2 Å due to due to natural thermal vibrations. Indeed, on the solid surface, Hads remains associated with a single surface Ga atom (bond length never exceeding 1.7 Å) from 5 ps onwards. In contrast, the liquid surface has Hads constantly moving. It regularly approaches within the 4 Å threshold of In, and comes as close as 2 Å at times. In the 5 to 25 ps window of the simulation, Hads on the liquid changes its closest bonded atom seven times, corresponding to a frequency of 0.35 bond changes per picosecond. An analogous picture is observed for Hads on Ga-Sn (see Supporting Information), where the adsorbate does not change bonds on the solid surface, but changes bonds 0.10 times per picosecond within the simulation window on the liquid surface.
Critically, while some of the motion of this Hads could be described as diffusing across Ga atoms, in other sections of the trajectory it either travels with a moving atom of liquid Ga along the surface, or is forced to move as the binding site it previously occupied rearranges. The liquid trajectory in Figure 3 shows the motion of Hads occurs mostly as gradual long-scale traversal over the liquid surface, appearing as contiguous regions (several picoseconds) of travel either towards or away form the dopant. As a result, we believe this motion of Hads on liquid surfaces is, to some extent, forced – in a way that is not observed on traditional solid materials.
Depending on whether one treats the adsorbate as constrained to a 2-dimensional plane or free to move in 3-dimensions, the average kinetic energy of a particle can be taken between and . The 160 K temperature difference between solid and liquid yields a only a very small difference in kinetic energy in the range of 0.01 to 0.02 eV. Because the energy difference in H adsorption sites spans a much larger range (up to around 0.60 eV)28 the difference in the motion of H is unlikely to be due to the temperature difference alone. This reinforces the idea that it is the motion of the liquid surface leading to Hads mobility.
The result of the enhanced mobility of surface Hads for the liquid system is that the dopant assisted pathway to CO2 reduction can be accessed through stochastic and passive motion of the system. Indeed, we suggest that any available surface H would eventually be moved into close contact with a dopant atom in the liquid. If a favourable approach of CO2 occurred while Hads was close to the dopant, this would allow the system to access the low barrier (around 0.25 eV) dopant assisted pathway to form formate readily. For the solid system, because the motion of H is not forced and its mobility is comparatively limited, it is far less likely for Hads to move from a favourable Ga site and approach the dopant. Therefore, once any Hads near the dopant atoms had reacted, it seems likely that the solid system is predominately limited to the dopant avoided pathway for CO2 reduction, with its much higher barrier (>0.50 eV). We argue that it is this effect, in conjunction with the existence of less dopant on the surface in the solid systems, that underpins the low CO2 reduction activity here. Naturally, the liquid systems are not limited in this way, and so low-barrier CO2 reduction can and does occur in high abundance.
Therefore, comparing the overall reaction between solid and liquid (Figure 4, showing Ga-In), it is clearly seen why CO2 reduction is favoured on the liquid surface. Based on the computational hydrogen electrode,29 the electrochemical adsorption of H will spontaneously occur ( between 0.26 and 0.35 eV) at the −1.16 V (versus RHE) applied potentials that Liu et al. report.12 Therefore, Hads will be passively present on both liquid and solid surfaces. The subsequent reaction with CO2 to form formate is non-electrochemical, and this barrier must be overcome thermally, making the lower dopant assisted barrier on the liquid highly impactful. Based on the barrier at this point, we estimate an upper limit for the current density towards CO2 reduction of 300 mA cm−2 (calculations included in the Supporting Information), which is slightly in-excess of the 10 mA cm−2 reported by Liu et al. at −1.16 V.12 However, given how sensitive the reaction rate is to small fluctuations of the activation barrier, this difference can be accounted for by a 0.1 eV shift in the activation energy. Although the formation of formate is a non-electrochemical step and therefore unlikely to be strongly affected by applied potential, a difference of this small magnitude could be the result of the computational model not including solvent or electrochemical counter-ions. Past work has suggested that CO2 can oxidise the Ga30, 31 or Sn32 in solids alloys as it undergoes dissociation. While we did not directly observe this process in our liquid models, the phenomenon may generate new active sites that are relevant to products along other branches of the CO2 reduction pathway (e.g. methanol). Future work could consider this possibility for accessing new products from liquid metal catalysts.
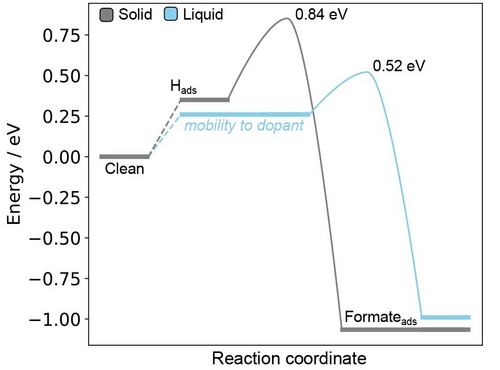
A reaction energy diagram comparing the overall processes for CO2 reduction on solid and liquid Ga-In. Note that, on the solid, the mobility of Hads is highly limited. Therefore the reaction to form formate will likely occur via a dopant avoided pathway (as shown). In contrast, on the liquid surface, Hads has enforced mobility with the motion of the surface and will passively drift close to a dopant atom. The reaction on the liquid can therefore occur via the dopant assisted pathway.
We have not studied the desorption of formate here, as this process would depend strongly on conditions such as the pH and solution environment. These factors were beyond the scope of computational feasibility for our models. However, this final step would be unlikely to differ greatly between solid and liquid – especially given that formate is similarly strongly bound to each.
In conclusion, metadynamics simulations have shown atomic-scale evidence for the reduction of CO2 occurring via a proton assisted pathway, with a strong preference for the reaction to occur at In and Sn dopant sites in Ga-alloys. This preference did not change when the alloys were liquid versus solid. However, surface Hads – which is required adjacent to a dopant to get this favourable pathway – was found to be far more mobile on the liquid surface. The constant rearrangement of the liquid structure indeed forced the ongoing motion of Hads. Therefore, we conclude that this mobility allows Hads to drift adjacent to dopant sites on the liquid surface, enabling low-barrier CO2 reduction in a way that could not readily occur on the solid. We argue that this is able to explain the highly selective CO2 reduction to formate that is observed on liquid Ga-alloys, but not solid. These findings underscore the idea that dynamic flexibility and constant atomic motion are critical to the unique activity of liquid metal catalysts. The inclusion of these factors in further studies on these fascinating materials is strongly advisable, especially when considering atomic-scale mechanisms.
Acknowledgments
The authors are grateful to The Royal Society of New Zealand and the MacDiarmid Institute for Advanced Materials and Nanotechnology for the funding to support this work. The computational resources were generously provided by the New Zealand e-Science Infrastructure. Open Access publishing facilitated by The University of Auckland, as part of the Wiley - The University of Auckland agreement via the Council of Australian University Librarians.
Conflict of Interests
There are no conflicts to declare.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




