Enantio- and Z-Selective δ-Hydroarylation of Aryl-Substituted 1,3-Dienes via Rh-Catalyzed Conjugate Addition
Abstract
Metal-catalyzed enantioselective conjugate arylations of electron-poor alkenes are highly selective processes for C(sp2)−C(sp3) bond formation. δ-Selective hydroarylations of electron-poor 1,3-dienes are less well developed and reactions that deliver high enantioselectivity while giving single alkene isomer products are elusive. Here we report the Rh-catalyzed δ-arylation of aryl-substituted 1,3-dienes that gives nearly exclusive Z-1,4-addition products (generally with >95 : 5 positional and geometrical selectivity). This remote functionalization provides access to chiral diarylated alkenes from readily available precursors poised for further functionalization, including in the synthesis of bioactive molecules. Mechanistic studies suggest that protonolysis of a Rh-allyl intermediate generated by diene insertion into a Rh-aryl is the turnover limiting step and occurs by an inner-sphere proton transfer pathway.
Catalytic enantioselective conjugate additions are one of the most useful and well-studied reactions for the stereocontrolled formation of C−C bonds.1 Among these processes, the Rh-catalyzed enantioselective hydroarylation of electron-poor alkenes is a leading method to generate new C(sp2)−C(sp3) stereocenters at the β-position relative to an electron-withdrawing group (Figure 1a).2 Along with providing products in high enantioselectivity, Rh-catalyzed conjugate additions occur under mild, weakly basic conditions frequently using bench-stable and readily available boronic acid derivatives. These reactions can accommodate a wide host of alkene acceptors including α,β-unsaturated esters, -ketones, -amides, and nitro- or sulfonylalkenes. Rh-catalyzed hydroarylations are routinely used in the synthesis of bioactive molecules,3 in medicinal chemistry programs,4 and on process-scales.5 Lam and co-workers comprehensive treatise supports the enormous diversity of substrates and synthetic applications of the reaction (>400 pages, >300 citations).6 While Rh-catalyzed hydroarylations have enjoyed rapid development since Hayashi and Miyaura's initial report in 1998,7 some valuable substrate classes, like aryl-activated substrates, remain challenging to use in enantioselective reactions. Because the alkene unit of an arylalkene is inherently less activated by the aryl substituent compared to a carbonyl and the resulting Rh-intermediates are prone to β-hydride elimination rather than protodemetalation,8 processes are limited to highly electron-deficient substrates like azaarenes9 or 4-nitroaromatics10 (Figure 1a).11, 12
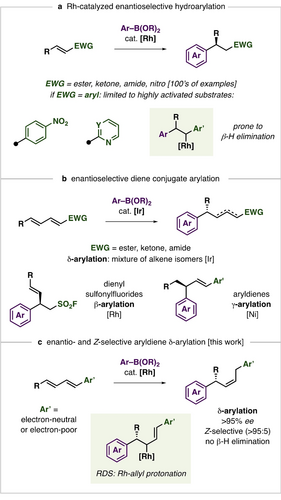
A) Overview and limitations of Rh-catalyzed enantioselective conjugate arylations. B) Established diene hydroarylation reactions using aryl boron nucleophiles. C) This work reports the Z-selective δ-arylation of aryl dienes which proceeds by turnover-limiting Rh-allyl protonation.
Electron-poor conjugated dienes represent attractive substrates for enantioselective metal-catalyzed conjugate addition because they can allow remote functionalizations relative to an activating group while the products bear an alkene for further functionalization.13 However, controlling the site of nucleophile addition (β vs δ) and generating single alkene regio- and stereoisomers is a challenge. With respect to metal-catalyzed δ-additions to linear dienes, examples are limited but include Cu-catalyzed alkylations14 or allylations,15 and Co-catalyzed alkynylations which each give E-alkene products.16 The hydroarylation of ester or ketone activated linear 1,3-dienes with aryl boroxines can be achieved with Ir-based catalysts, however a mixture of positional and geometric isomers are generated and products of the reactions are typically isolated after isomerization to the α,β-unsaturated species or alkene hydrogenation (Figure 1b).17 Sulfonyldienes have been shown to undergo Rh-catalyzed hydroarylation at the β-position where the selectivity arises from the strongly electron-withdrawing nature of the SO2F activating group.18 Aryl-substituted 1,3-dienes have been reported to undergo Ni-catalyzed γ-arylation reported with selectivity rationalized by protonation (or hydride addition) to the least sterically hindered site of the diene substrate which varies based on the size of the arylboronic ester unit.19 Wang and co-workers recently disclosed the Ni-catalyzed Z-selective δ-arylation of terminally unsubstituted aryl 1,3-dienes.20 Given the state-of-the-art of enantioselective diene conjugate additions, it would be valuable to identify more general, mechanistically understood processes for δ-arylations that give single alkene isomer products. This reaction would enable the synthesis of compounds with remote stereocenters difficult to prepare by known methods while possessing an olefin handle for further synthetic elaboration.
We have developed catalytic Z-selective additions to electron-poor 1,3-dienes, including transfer hydrogenations,21 reductive couplings with aldehydes,22 and enantioselective multicomponent couplings.23 In these processes, intercepting the Rh-allyl intermediate generated after alkene insertion in a controlled fashion and at a rate faster than isomerization was key to observing high regio- and stereoselectivities. Armed with this understanding we questioned whether weakly activated aryl-substituted 1,3-dienes could undergo enantioselective hydroarylation and herein document the development, scope, application, and mechanistic features of such processes (Figure 1c).
With the aim of developing an enantioselective δ-arylation of diene substate 1, a range of privileged ligands for conjugate addition and various reaction conditions were surveyed (Figure 2a). It was ultimately found that a Rh-catalyst supported by Nishimura and Hayashi's tetrafluorobenzobarrelene ligand, Ph-tfb,24 provided excellent results, giving 2 in 87 % yield, 98 % ee, and >95 : 5 Z : E. While the ferrocenyl tfb-ligand analog also gave good results (87 % yield, 98 % ee), alkylated versions of tfb (Bn or Me) or structurally similar bicyclo[2.2.2]octadienes (L1–L3) gave product in reduced yields. Use of the less electron-poor, phenyl-substituted ligand L2 provided similar ee as Ph-tfb, but with slower rates. The use of MeOH as the reaction solvent was important. Using solvent mixtures common for Rh-catalyzed conjugate additions where the protic additive was diluted with co-solvents like dioxane, toluene, DMF resulted in lower yields (Figure 2b, entries 2–5). Aryl boronic pinacol esters could be used instead of the corresponding boronic acid with nearly identical results (entry 8). This is useful in cases where substrates undergo fast protodeborylation (see below). Ir-based catalysts gave 2 in lower yield but with good ee (41 %, 92 % ee, entry 9). The reaction is not limited to electron-poor dienes. With minor modification to reaction conditions, a phenyl substituted diene derived substrate underwent enantioselective δ-arylation in 70 % yield and 99 % ee (entry 10). Finally, 3,4-hydroarylation products can be readily generated from the standard 1,4-addition products by treatment with base to isomerize the olefin into conjugation with the Ar’ unit without erosion of enantioselectivity (Figure 2c, 3, 91 % yield, 98 % ee).
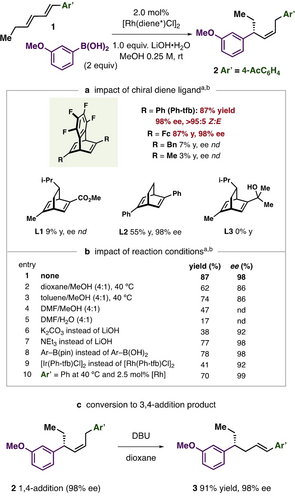
Reaction Development. A) Impact of ligand structure on reactivity and selectivity. B) Effect of reaction conditions. C) Synthesis of 3,4-hydroarylation product. [a] Yields determined by 1H NMR spectroscopy using trimethoxybenzene as an internal standard, Z : E are >90 : 10 in all cases. [b] ee determined by chiral HPLC after alkene epoxidation, see Supporting Information for details.
With a selective δ-arylation process discovered, mechanistic studies were conducted to probe the origin of desirable reactivity and to contrast with well-established Rh-catalyzed alkene β-arylations. The order of the reaction with respect to reactants and catalyst was determined by reaction progress kinetic analysis variable time normalization plots25 of the reaction between diene 1, 3-methoxyphenyl pinacol boronic ester and [Rh(Ph-tfb)Cl]2 to generate 2. The reaction displayed overall zero order kinetics, with some small deviation as full substrate conversion is achieved. The process was found to be zero order in both diene and aryl boronic ester while being first order in catalyst (Figure 3a, see Supporting Information for details). The reaction is approximately zero order in base (LiOH ⋅ H2O), however slightly better overlays are obtained with considering the order to be −0.3 (see Supporting Information for plots).
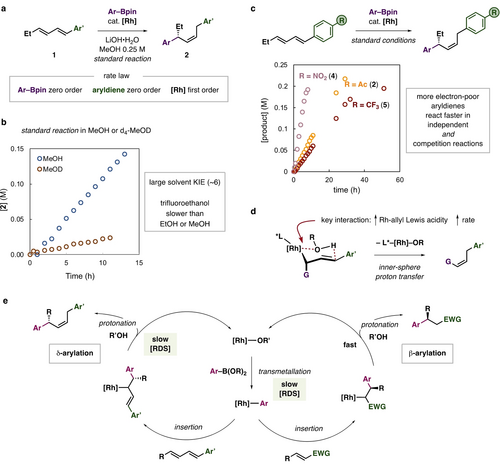
Mechanistic studies. A) Rate law overview determined by variable time normalization plots. B) Solvent kinetic isotope effect. C) Reaction rates of differentially substituted dienes. D) Proposed structure required for Rh-allyl protonolysis. E) Mechanistic proposal and comparison to alkene conjugate arylation. Unless noted Ar=3-OMeC6H4, Ar’=4-AcC6H4.
The standard δ-arylation reaction to form 2 exhibited a large primary H/D kinetic isotope effect of ~6 when comparing reactions conducted in MeOH vs d4-MeOD (Figure 3b). This KIE value is likely an underestimate as some protonated product is observed when using d4-MeOD due to the presence of LiOH ⋅ H2O. Increasing the Bronsted acidity of the solvent by replacing methanol with 2,2,2-trifluoroethanol decreased reaction rates. More electron-poor dienes underwent faster reactions, demonstrated by a series of 4-aryl substituted substrates (Figure 3c; NO2, Ac, and CF3). In competition studies between diene substrates, the more electron-poor aryl-substituted 1,3-diene underwent faster reaction but did not inhibit conversion of the more electron-rich diene (see Supporting Information for details). Reactions using d4-MeOD suggest Rh-allyl protonation is diastereoselective as product d1-5 was generated with high diastereoselectivity. D-incorporation is observed exclusively at single site at the allylic methylene position (D-incorporation is observed at multiple positions in 2 due to base promoted exchange, see Supporting Information for details). The presence of Lewis bases (pyridines, amines) tends to slow the reaction down. Collectively, these results suggest the rate of protonation of the nucleophilic Rh(I)-allyl intermediate is governed by the Rh-center's Lewis acidity and the species responsible for protonation (i.e. MeOH) likely coordinates to Rh prior to proton transfer (Figure 3d). This proposal agrees with the need for more electron-withdrawing tfb-type ligands compared to structurally similar bicyclo[2.2.2]octadiene ligands. The high observed regio- and stereochemistry of the Z-alkene in the resulting products can be rationalized by a highly ordered transition state akin to that proposed for the Z-selective allylrhodation of aldehydes.22, 26
Overall, mechanistic data combined with the established steps of Rh-catalyzed alkene conjugate addition reactions leads to a plausible mechanism where the turnover limiting step is the protonolysis of a Rh-allyl intermediate by methanol (Figure 3e). This step occurs after transmetallation to generate a Rh-aryl intermediate which can undergo insertion at the 4-position of the diene substrate. The kinetics of diene hydroarylation contrast that of enone hydroarylation, where the reaction is half-order in Rh-catalyst and positive order in arylboronic acid with transmetallation proposed as the turnover limiting step (Figure 3e).27 The facial selectivity of diene δ-arylation is the opposite that of alkene β-arylation.28 The mechanistic differences between Rh-catalyzed conjugate additions to enones and dienes help to explain why mixtures of alkene isomers are commonly generated in previously developed diene hydroarylation reactions. If the Rh-allyl species is long-lived because protonation is slow, isomerization is likely. This step needs to be promoted with a combination of a more electrophilic Rh-center and an appropriate solvent to realize a productive reaction. These findings should have more general application in the design of stereo- and site-selective metal-catalyzed conjugate addition reactions.
The Rh-catalyzed Z- and δ-selective hydroarylation reaction can accommodate a host of aryl substituted 1,3-diene substrates (Figure 4), including those with electron-withdrawing groups (Ac, NO2, CF3, CN, SO2Me; 2, 4–7), pyridyl-containing diene 10, and those with a simple phenyl group (8, 17) to give products in good to moderate yields and generally ≥94 % ee. 2 could be prepared on a 1.2 gram scale in 88 % yield and 98 % ee. The δ-position of diene substrate where the new stereocenter is formed could bear a host of groups including Me and i-Bu units (16, 15), N-Boc amines (11), alkyl chlorides (12), nitriles (13), and protected alcohols (14) to give δ-arylation products in ≥95 % ee. Steric bulk is tolerated adjacent to the reactive δ-position in the aryl 1,3-diene substrate without competing β-arylation (38). Less successful examples include electron-rich dienes like (4-methoxyphenyl)butadiene and 1,4-diarylbutadiene derivatives. Diene substrates were generally prepared by a Sonogashira/alkyne isomerization sequence29 which gave products in ~95 : 5 E,E/E,Z. Conveniently, the E,Z-diene isomers are inert towards hydroarylation and do not impact the reaction so the geometric purity of diene substrate is inconsequential. Of the diene substrates explored, positional selectivity (δ vs β), alkene regioselectivity, and E/Z-selectivities each generally remained >95 : 5. Exceptions are phenyl substituted diene derived products 8 and 17 where ~10 % δ-arylated styrene is observed due to post-arylation base-mediated alkene isomerization and the highly activated 4-NO2 product 4 which is obtained in 89 : 11 Z/E.
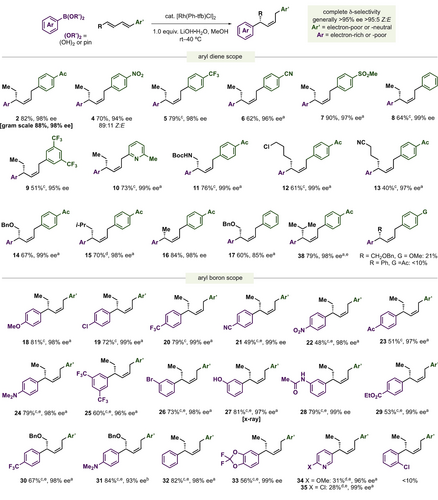
Reaction scope of dienes and aryl boron reagents. Ar=3-OMeC6H4, Ar’=4-AcC6H4 Diene : ArB(OR)2=1 : 2–3, unless noted using 2 mol % [Rh] dimer and the corresponding boronic acid. Unless noted, yields are of isolated material and ee determined by chiral HPLC after alkene epoxidation. [a] ee determined without derivatization. [b] ee determined after alkene hydrogenation. [c] Using 2.5 mol % [Rh] dimer. [d] Using 5 mol % [Rh] dimer. [e] Using the corresponding pinacol boronic ester.
Aside from ortho-substitution, the scope of aryl boron partners had no significant limitations. Reactions proceed with good yields and ≥93 % ee for electron-rich (18, 24, 27, 28, 31), -neutral, (32) and -poor (20, 21, 22, 23, 25, 29, 30) aryl boron substrates. The reaction tolerates aryl bromides (26), basic amines (24, 31), unprotected phenols (27), and NHAc groups (28). 3-Pyridyl containing stereocenters can be generated with high ee (96–99 %) although with reduced yields (34, 35). The corresponding aryl boronic pinacol esters were used in examples 21, 22, and 24–35 due to their improved resistance to protodeboronation. In general, Lewis-basic pyridyl and cyano groups were found to impede the reaction but not completely inhibit it (see Supporting Information for details and other unsuccessful substrates).
Enantioselective diene δ-additions can be used to prepare intermediates of compounds that have biological interest in a straight-forward fashion (Figure 5). Compound 36, a key intermediate in the synthesis of a sphingosine-1 phosphate receptor modulator (P1),30 was synthesized by cross-metathesis of an allylbenzene and vinylBpin, followed by Suzuki coupling, Rh-catalyzed δ-hydroarylation and hydrogenation in 39 % overall yield and 94 % ee (Figure 5). Suzuki coupling of an amino vinyl boronic ester and β-bromostyrene followed by enantioselective diene δ-hydroarylation and hydrogenation provides 37 (59 % overall yield, 96 % ee), an intermediate in the synthesis of inhibitor of human methionine aminopeptidase-1 (P2, Figure 5).31 These targets were previously prepared as racemic mixtures. Slight erosion of ee (~2 %) was observed upon alkene hydrogenation, presumably due to the sensitive nature of the benzylic stereocenter (see Supporting Information for details).
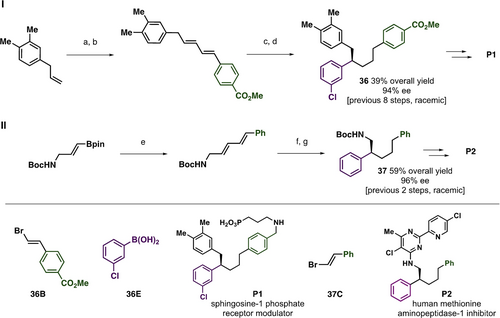
Synthetic applications of Rh-catalyzed enantioselective δ-hydroarylation, see the Supporting Information for full details. I) a) 2 equiv. vinylB(pin), 5 mol % Grubbs G1, CH2Cl2 50 °C, 60 % yield; b) 1 equiv. 36B, 3 mol % Pd2dba3, 15 mol % AsPh3 aq. KOH, THF, rt, 80 % yield; c) 3 equiv. 36E, 2 mol % [Rh((S,S)-Ph-tfb)Cl]2, MeOH 40 °C, 90 % yield, 96 % ee; d) 5 mol % Crabtree's catalyst, 50 atm H2, CH2Cl2, 90 % yield, 94 % ee. II) e) 1 equiv. 37 C, 3 mol % Pd2dba3, 15 mol % AsPh3 aq. KOH, THF, rt, 82 % yield; f) 3 equiv. PhB(pin) 2 mol % [Rh((S,S)-Ph-tfb)Cl]2, MeOH 40 °C, 75 % yield, 99 % ee; g) 5 mol % Rh(PPh3)3Cl, 1 atm H2, MeOH rt, 95 % yield, 96 % ee.
We have established the Rh-catalyzed δ-selective hydroarylation of aryl substituted 1,3-dienes. The process gives Z-alkene containing products often in >95 % ee and does not require strong electron-withdrawing groups on the diene unit while tolerating protic and electrophilic groups on the aryl boronic acid partner. Mechanistic studies suggest that inner-sphere proton transfer in a Rh(allyl)(methanol) complex is the turnover limiting step and enhancing Rh Lewis acidity using an electron-poor chiral diene ligand imparts faster and more selective reactions. These findings should have use in related remote, enantioselective C(sp2)−C(sp3) bond formations and the design of site-selective metal-catalyzed conjugate-type additions to polyunsaturated substrates.
Acknowledgments
We thank NSERC Canada (RGPIN-2019-06050 and RGPAS-2019-00051 to R. J. L., PGS-D to C. J. C. C.), the University of Alberta, and the Province of Alberta (AGES fellowship to C. J. C. C.) for support. Dr. Michael Ferguson (Alberta) is thanked for X-ray crystal structure determinations, Wesley McNutt is thanked for helpful discussions and Qiqige Qiqige is thanked for assistance with catalyst preparation.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




