Bridged Pt−OH−Mn Mediator in N-coordinated Mn Single Atoms and Pt Nanoparticles for Electrochemical Biomolecule Oxidation and Discrimination
Abstract
The rational design of efficient catalysts for uric acid (UA) electrooxidation, as well as the establishment of structure-activity relationships, remains a critical bottleneck in the field of electrochemical sensing. To address these challenges, herein, a hybrid catalyst that integrates carbon-supported Pt nanoparticles and nitrogen-coordinated Mn single atoms (PtNPs/MnNC) is developed. The metal-metal interaction during annealing affords the construction of metallic-bonded Pt−Mn pairs between PtNPs and Mn single atoms, facilitating the electron transfer from PtNPs to the support and thereby optimizing the electronic structure of catalysts. More importantly, experiments and theoretical calculations provide visual proof for the ‘incipient hydrous oxide adatom mediator’ mechanism for UA oxidation. The Pt−Mn pairs first adsorb OH* to construct the bridged Pt−OH−Mn mediators to serve as a highly active intermediate for N−H bond dissociation and proton transfer. Benefiting from the unique electronic and geometric structure of the catalytic center and reactive intermediates, PtNPs/MnNC exhibits superior electrooxidation performance. The electrochemical sensor based on PtNPs/MnNC enables sensitive detection and discrimination of UA and dopamine in serum samples. This work offers new insights into the construction of novel electrocatalysts for sensitive sensing platforms.
Introduction
Small biomolecules, such as uric acid (UA), play a pivotal role in the immune response and organism maintenance,1 and their disruptions in biomolecule levels usually lead to metabolic imbalances and even autoimmune diseases.2 Consequently, their detection carries significant importance in terms of disease prevention, early diagnosis, and effective treatment.3 Electrochemical sensors that integrate functional nanomaterials featuring adjustable structures and rapid electrochemical responsiveness are considered promising benchmarks.4 Despite their effectiveness, the sensing application of these nanomaterials remains an ongoing issue due to their less-than-unsatisfactory sensitivity and limited detection range, which primarily arise from their insufficient intrinsic activity and electron transfer capabilities.3b, 5
Electrochemical detection of UA generally involves the UA adsorption and dissociation of N−H bonds induced by electron polarization.6 According to the widely established ‘incipient hydrous oxide adatom mediator’ (IHOAM) models for metal catalyst-based electrooxidation, the active sites are proposed to undergo a pre-oxidation step by hydroxyl (−OH) to form the metal−OH species, which can serve as a “mediator” for subsequent oxidation.7 In this regard, the oxidation reaction kinetics are highly related to the interaction between metal sites and −OH as well as the reactivity of this metal−OH mediator, which can manipulate the electron polarization of the N−H bond and the removal of the dehydrogenation product. To date, although various catalysts have been reported for the electrooxidation of UA, the rational modulations of their electronic and geometric structures to satisfy adsorption/desorption balance and establish structure-activity relationships remain challenges. More importantly, the adsorption/desorption seesaw issues are still difficult to solve over individual sites.3b, 8 To this end, endeavors to design unique catalytic centers with optimal configurations to facilitate the −OH adsorption and efficient H withdrawal from UA are the subjects of interest and priority.9
Herein, we design a hybrid catalyst consisting of carbon-supported PtNPs and nitrogen-coordinated Mn single atoms (SAs) (named PtNPs/MnNC) to boost the electrooxidation performance of biomolecules. Specifically, the metallic-bonded Pt−Mn interaction is observed between Mn SAs and PtNPs, which strengthens the electron transfer from PtNPs to support and thus optimizes the electronic structure of the catalytic center. More importantly, experimental and theoretical investigations first provide the visualized evidence for the successful construction of a bridged Pt−OH−Mn mediator during the catalytic procedure, showing much higher reactivity than traditional Pt−OH mediator. Leveraging the direct coupling effect between Pt and Mn sites, as well as the innovative bridged Pt−OH−Mn reactive intermediates, the highly accurate detection and discrimination of UA and dopamine (DA) were achieved even in complex interference systems. This finding paves the way for the development of efficient catalysts for sensitive electrochemical sensors.
Results and Discussion
MnNC was first obtained by annealing glucosamine and Mn precursor within Zn and silica colloidal spheres as dual templates.10 Subsequently, an impregnation-reduction procedure was conducted to obtain PtNPs/MnNC (Figure 1a).11 As a comparison, nitrogen-doped carbon (NC) was obtained without the addition of Mn precursor, and PtNPs were further introduced to yield PtNPs/NC. Transmission electron microscopy (TEM) images reveal the rich mesoporous and defect structures for all catalysts and abundant NPs in PtNPs/MnNC and PtNPs/NC (Figures 1b and S1–S3). Further investigations through the high-angle annular dark-field (HAADF) scanning TEM (STEM) images show that PtNPs are surrounded by abundant Mn SAs in PtNPs/MnNC (Figure 1c–d). Notably, the pure Pt crystalline structure with lattice distances of 0.211 nm for the (111) plane is confirmed in the region of PtNPs, which is consistent with the fast Fourier transform result and Rietveld refinement of X-ray diffraction (XRD) patterns (Figures S4–S5).12 In contrast, the line scan profile of PtNPs/MnNC reveals that except for Pt, increased Mn and N elements are also observed in the region of PtNPs compared to the adjacent support (Figure S4c). These results illustrate that the integration of PtNPs triggers the migration of Mn SAs to approach PtNPs, which is expected to achieve a strong interaction between Pt and Mn.10b, 13 The Pt loadings both of PtNPs/NC and PtNPs/MnNC are identified as ~3.79 wt % and ~3.86 wt %, respectively. Moreover, isothermal N2 adsorption/desorption and Raman spectra reveal that the PtNPs/MnNC and control samples show rich surface area with considerable porosity and defect structures, which provide ample exposed sites for small molecule adsorption and mass transport (Figures S6–S7 and Table S1).14
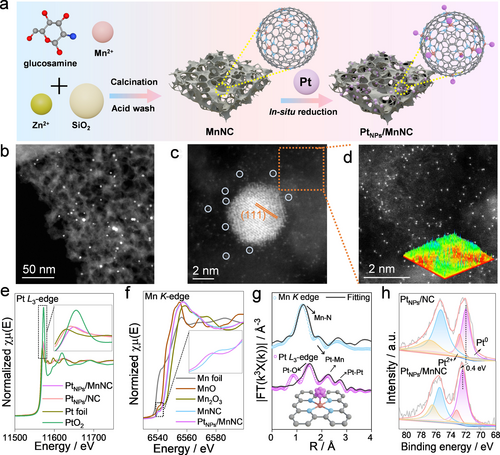
(a) Schematic illustration of the preparation of PtNPs/MnNC. (b) Low magnification HAADF STEM images of PtNPs/MnNC. High magnification HAADF STEM images in the region of (c) PtNPs and (d) carbon substrate of PtNPs/MnNC. Inset in panel (d) is the corresponding intensity profile image of gray levels. (e) XANES spectra on Pt L3-edge of PtNPs/MnNC, PtNPs/NC and standard samples (Pt foil and PtO2). The inset is an enlarged view of the white-line region. (f) XANES spectra on Mn K-edge of MnNC, PtNPs/MnNC, and standard samples (Mn foil, Mn and Mn2O3). The inset is an enlarged view of the dashed box region. (g) EXAFS spectra and corresponding fitting curves of PtNPs/MnNC on Mn K-edge and Pt L3-edge and proposed models of PtNPs/MnNC. The fitting range is from 1 to 3 Å. (h) Deconvoluted XPS Pt 4 f spectra of PtNPs/MnNC and PtNPs/NC.
X-ray absorption near-edge spectra (XANES) were employed to explore the valence state and chemical environment of catalysts. As shown in Figure 1e, Pt-L3 XANES spectra of PtNPs/MnNC and PtNPs/NC are close to that of Pt foil, emphasizing the metallic state of Pt. Moreover, compared to PtNPs/NC, the positively shifted white-line peak of PtNPs/MnNC suggests a more electron-deficient state after the introduction of Mn SAs, which can be further verified by the first derivative results on the Pt L3-edge (Figure S8).15 In contrast, the Mn K-edge XANES spectrum of PtNPs/MnNC shows a negative shift in comparison to MnNC, indicating the electron transfer from PtNPs (Figure 1f). The reduced MnN4 square planar D4h symmetry feature of PtNPs/MnNC (dashed box marked) relative to MnNC suggests the stretched Mn−N bond induced by PtNPs.16 Furthermore, the coordinated structure was verified by the extended X-ray absorption fine structure (EXAFS) oscillation. As shown in Figure 1g, the Mn K-edge EXAFS spectrum displays that PtNPs/MnNC delivers two prominent peaks at ~1.5 Å and ~2 Å. The first shell originates from the Mn−N scattering path with the coordination numbers of 4 based on the fitted results, and the latter one can be assigned to the Mn−Pt contribution. While MnNC only shows one prominent peak originating from the Mn−N contribution (Figure S9 and Table S2). For Pt L3-edge EXAFS spectra, besides the major peak of Pt−Pt between 2.5–3 Å, one shoulder peak located at ~2 Å can also be observed compared to PtNPs/NC, strongly illustrating the production of Pt−Mn interaction in PtNPs/MnNC (Figure S10 and Table S3).17 Besides, the poor fit results when the Pt−Mn pathway is excluded from the fitting model further confirm the validity of the Pt−Mn contribution in PtNPs/MnNC (Figure S11). Combined with the above results, it can be concluded that the metal-metal interaction during the pyrolysis process affords the dynamic migration of metals and the production of metallic-bonded Pt−Mn pairs, consistent with the TEM results.17 The possible Pt−Mn interaction can be further corroborated by wavelet transform (WT) contour plots (Figure S12). In detail, the warp of Mn K-edge WT-EXAFS in PtNPs/MnNC at ~5 Å relative to MnNC, and the distinct extension of WT maximum of Pt L3-edge WT-EXAFS plot for PtNPs/MnNC before the Pt−Pt path (~7.5 Å) compared to PtNPs/NC suggest the signal disturbance derived from Pt−Mn interaction. Additionally, the electronic interaction was further studied through X-ray photoelectron spectroscopy (XPS). The XPS survey confirms the existence of C, N, and O for all catalysts (Figure S13). The appearance of the additional peak in deconvoluted XPS N 1s spectra of PtNPs/MnNC and MnNC relative to PtNPs/NC and NC is attributed to MnNx moieties (Figure S14). Particularly, the positive shift of Pt0 species in the Pt 4 f spectrum of PtNPs/MnNC compared to PtNPs/NC reveals the electron transfer from PtNPs to support, which can be further verified by the C 1s spectra of catalysts and matches well with XANES results (Figures 1h and S15). By this token, the dynamic migration process during annealing not only effectively leads to the unique geometric structure between Pt and Mn, but also induces strong electronic interactions between PtNPs and support, which may endow PtNPs/MnNC with high catalytic activity.
DA and UA molecules are selected as the models to investigate the biomolecule oxidation performance of catalysts as they are considerably crucial biomarkers (Figure 2a).3b Before the evaluation of catalytic properties, the charge transfer characteristics of catalyst-modified electrodes were first compared. The improved redox current response towards [Fe(CN)6]3−/4− probe and a small semicircular portion at high frequency in Nyquist plot of PtNPs/MnNC-modified electrode compared to bare glassy carbon electrode indicate its improvement in the reaction kinetics (Figures S16–S17).18 The wide potential windows (~2.50 V) according to linear sweep voltammetry curves ensure that there are no interfering reactions during the UA and DA oxidation (Figure S18).
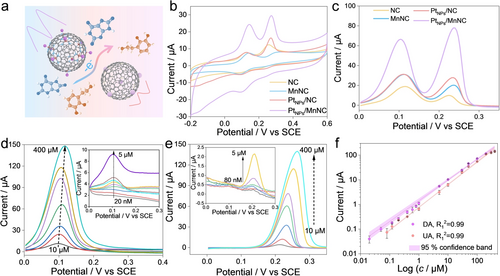
(a) Scheme of electrochemical oxidation of DA and UA. (b) CV curves and (c) DPV curves of PtNPs/MnNC, PtNPs/NC, MnNC, and NC by adding 100 μM DA and 100 μM UA in 0.1 M PBS (pH=7.4). DPV responses of PtNPs/MnNC in 0.1 M PBS (pH 7.4) by individually adding different concentrations of (d) DA and (e) UA. (f) Calibration curves of PtNPs/MnNC for DA and UA detection. All error bars mean±SD calculated from three independent measurements.
Then, the electrochemical oxidation capabilities of catalysts were compared through cyclic voltammetry (CV) and differential pulse voltammetry (DPV) responses in 0.1 M PBS (pH=7.4) containing mixtures of 100 μM DA and 100 μM UA. CV curves shown in Figure 2b display two well-defined DA and UA oxidation peaks at potentials of ~100 and 250 mV with a large separation of peak potentials (ΔE, 150 mV). Also, PtNPs/MnNC achieves the best performance for the detection of DA and UA, indicating the intrinsic superiority of the PtNPs/MnNC after the integration of Mn SAs and PtNPs (Figures 2c and S19). More importantly, the remarkable elevation of oxidation current for PtNPs/MnNC in comparison to PtNPs/NC and MnNC powerfully manifests the synergistic effect between PtNPs and Mn SAs.
As displayed in Figure 2d–e, PtNPs/MnNC delivers significant DPV responses for the successive addition of DA or UA. Accordingly, the linear relationships of the calibration curves for DPV peak currents versus DA or UA concentrations were established (Figure 2f). The sensitivities are calculated as 0.86 μA/μM and 0.90 μA/μM with the limit of detection (LOD, 3 s/k) of 4.88 nM and 13.67 nM for DA and UA, respectively. Compared with the reported catalysts listed in Table S4, PtNPs/MnNC shows outstanding DA and UA detection performance in terms of LOD and linear range.19
Based on the above discussion, PtNPs/MnNC delivers obvious superiority for biomolecule oxidation. Density functional theory (DFT) calculations were implemented to reveal the synergistic interaction between Mn SAs and PtNPs, and UA oxidation was chosen as the model to provide an in-depth investigation during the reaction. Specifically, carbon-supported four N atoms coordinated Mn single atom was used to simulate MnNC, and MnNC or NC coupled with pyramid-like Pt14 cluster were to PtNPs/MnNC and PtNPs/NC, respectively (Figure S20).20 One Pt atom is bonded to one Mn atom to construct a metallic bonded Pt−Mn pair based on the EXAFS results. Furthermore, more electron transfer from PtNPs to support was observed after the integration of Mn SAs according to the charge density difference analysis, suggesting the reinforced electronic interaction (Figure S21). As a result, PtNPs/MnNC exhibits an upshifted d-band center (−1.76 eV) compared to PtNPs/NC (−1.98 eV), which is expected to deliver enhanced oxidation capacity (Figure 3a).21
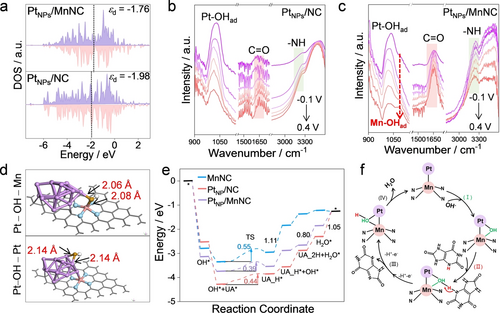
(a) Projected density of state diagrams of PtNPs/MnNC and PtNPs/NC. In situ ATR-FTIR spectra of (b) PtNPs/NC and (c) PtNPs/MnNC under different potentials within adding UA. (d) Different adsorption models of *OH on PtNPs/MnNC. (e) Free energy profiles of UA oxidation for PtNPs/MnNC, PtNPs/NC and MnNC. (f) Proposed reaction process on PtNPs/MnNC.
In situ attenuated total reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR) was further employed to provide an in-depth investigation. As depicted in Figure S22, in PBS solution without adding UA, the gradually increased characteristic peak at 1,040 cm−1 for PtNPs/NC and PtNPs/MnNC over the increased potential can be assigned to the adsorbed -OH species on the Pt sites.22 Specifically, PtNPs/MnNC shows a more obvious increased tendency than PtNPs/NC. Moreover, compared to PtNPs/NC, one group of peak at 1,090 cm−1 appears for PtNPs/MnNC, which can be attributed to Mn−OH signals.23 These results illustrate that Pt sites can undergo a pre-oxidation process, while Mn SAs in PtNPs/MnNC not only enhance the adsorption of −OH at the Pt sites but also produce Mn–OH intermediates to participate in the pre-oxidation, which may facilitate the oxidation reaction. After the injection of UA, the peaks at 1,652 cm−1 and 3,243 cm−1 correspond to the C=O and N−H vibrations, respectively, from the adsorbed UA molecules (Figure 3b–c). The higher peak intensities of PtNPs/MnNC than PtNPs/NC demonstrate that more UA molecules were adsorbed on PtNPs/MnNC with the assistance of Mn sites. Notably, the Pt−OH peak was observed to decrease over the increased potentials, demonstrating that the adsorbed −OH was consumed during UA oxidation. In this regard, it is rational to propose that the UA oxidation on the resultant catalysts follows the IHOAM models, where metal−OH species serve as the reactive intermediates to conduct the following oxidation procedure.24
The role of Mn SAs on UA oxidation was then explored in detail. As shown in Figure S23, both MnNC and PtNPs/NC exhibit a weak affinity for −OH, indicating their relatively low catalytic performance. Upon the introduction of Mn SAs, PtNPs/MnNC exhibits enhanced adsorption strength for −OH. Besides, compared to adsorbing individually on Pt sites, −OH prefers to undergo “bridge adsorption” in thermodynamics to construct a bridged Pt−OH−Mn site. The bond length of Pt−OH and Mn−OH in the bridged Pt−OH−Mn site is shorter than individual Pt−OH sites (2.06 and 2.08 versus 2.14 Å), stressing the enhanced adsorption strength towards −OH derived from the evolution of bridged Pt−OH−Mn site (Figure 3d). Combined with in situ ATR-FTIR results, it is believed that Mn SAs not only regulate the electronic structure of catalysts but also serve as the additional adsorption site to participate in the oxidation reaction. Free energy profiles were obtained to evaluate the UA oxidation performance of different catalysts. As shown in Figure S24, the electrochemical UA oxidation typically follows a two-electron two-proton transfer process and a regeneration step occurs to produce new M−OH species for the second electron and proton transfer process. As shown in Figure 3e, MnNC delivers inferior UA oxidation performance because of the high transition state energy barrier for the first H-withdrawing step. Taking advantage of highly active bridged Pt−OH−Mn reactive intermediates, PtNPs/MnNC shows much lower transition state energy (0.39 eV) than PtNPs/NC (0.44 eV) for the N−H broken. What's more, the rate-determining step (H2O* desorption) of PtNPs/NC is greatly relieved in PtNPs/MnNC, indicating that the combination of Pt and Mn sites unlocks the adsorption/desorption seesaw of individual active sites. Overall, due to the electronic interaction and the unique bridged Pt−OH−Mn mediator, PtNPs/MnNC demonstrates significantly enhanced activity for the electrooxidation of biomolecules (Figure 3f).
Given that DA and UA present similar structures and redox potentials and commonly coexist in the human body, their sensitive detection and selective identification without mutual interference are significant for diagnostic research and pathological monitoring.3a As shown in Figures 4a and S25, when fixing UA concentration, the oxidation current of DA gradually increases with its successive addition, while the oxidation current of UA remains constant. Conversely, with a fixed DA concentration, the DPV current of UA gradually rises and the peak of DA remains unchanged. This indicates that PtNPs/MnNC can effectively detect one substrate in the presence of the other one. More importantly, the increase in oxidation currents both for DA and UA are observed with their simultaneous addition. Combined with the calibration curves, PtNPs/MnNC enables simultaneous detection of DA and UA with little mutual interference of the current signals (Figure 4b). Furthermore, the detection of DA and UA within three catalysts (PtNPs/MnNC, PtNPs/NC, and MnNC) was performed to establish the training matrix for discriminating DA and UA.25 As depicted, two separate clusters corresponding to DA and UA indicate the success of their identification and discrimination, and the short distances among six parallel classes in each cluster demonstrate high accuracy and good reproducibility (Figure 4c). Even in the simultaneous presence of DA and UA, they can still be clearly distinguished, and these cluster positions are almost identical to those when they exist individually, suggesting that our proposed biosensor can achieve high sensitivity and precision in the recognition of UA and DA.
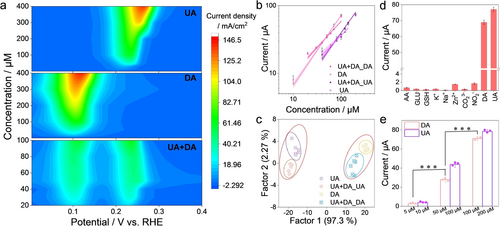
(a) Contour Figures according to the DPV responses of PtNPs/MnNC by dividually or simultaneously adding different concentrations of DA and UA in 0.1 M PBS (pH=7.4). (b) Calibration curves of PtNPs/MnNC towards DPV peak currents versus substrate concentrations for individual (dot, fixing concentration of another substrate) and simultaneous (triangle) detection of DA and UA, respectively. (c) Linear discriminant analysis plot for discrimination of DA and UA. (d) Selectivity of PtNPs/MnNC towards DA and UA detection. (e) Peak currents of DPV responses of PtNPs/MnNC to detect DA and UA in serum samples. All error bars mean±SD calculated from three independent measurements, *** p<0.001. Statistical significance is assessed by the Student's two-tailed test.
As for a practical sensing application, selectivity, anti-interference ability, and stability are also crucial indicators. As shown in Figure 4d, injecting various common small molecules, metal ions, or anions in biological systems does not yield significant electrochemical signals. The introduction of these interferents during the detection of UA and DA has no impact on their DPV currents (Figure S26). These findings demonstrate that the constructed electrochemical sensor exhibits exceptional selectivity and interference resistance. Meanwhile, the stable current responses of repeated detection for DA and UA over several days indicate the excellent stability of the PtNPs/MnNC-based sensing platform (Figure S27). Taking advantage of the high sensitivity, selectivity, and stability of PtNPs/MnNC, their practical potential was further investigated in serum samples through the standard addition method. As expected, the recovery of UA and DA ranges from 97.27–103.76 % and 101.43–104.58 %, respectively, indicating that PtNPs/MnNC-based electrochemical sensors can be effectively used for the detection of DA and UA in the real biological sample (Figures 4e and S28).
Conclusion
In conclusion, our work achieves highly efficient biomolecule oxidation through the construction of a catalyst composed of carbon-supported Pt nanoparticles and nitrogen-coordinated Mn single atoms (PtNPs/MnNC). The direct coupling effect between Pt and Mn within the formation of metallic-bonded Pt−Mn pair results in the encouraged electron transfer and optimized electronic structure of Pt sites. More importantly, the bridged Pt−OH−Mn mediators constructed during the reaction function as active reactive intermediates following the IHOAM models, accelerating N−H bond dissociation and proton transfer for uric acid oxidation. Taking advantage of the above modulation results, the noteworthy improvement in electrooxidation performance demonstrated by PtNPs/MnNC achieves its sensitive detection and discrimination of biomolecules in complex biological matrices, which underscores its practical potential for an electrochemical sensing platform. The rational design of nanocatalysts for electrooxidation and the in-depth exploration of their catalytic mechanisms in this work present significance for the construction of highly efficient and sensitive electrochemical sensors.
Acknowledgments
The authors gratefully acknowledge the financial support from a start-up fund of Central China Normal University, the Fundamental Research Funds for the Central Universities (no. CCNU22JC006), the Program of Introducing Talents of Discipline to Universities of China (111 program, B17019), and the JST-ERATO Yamauchi Materials Space-Tectonics Project (JPMJER2003) and the ES Program (via Nagoya University). We thank Prof. Weiyu Song and Shaojia Song of China University of Petroleum for their help in theoretical calculation. We thank Dr. Xiaoli Cai from Wuhan University of Science and Technology for providing the serum sample. We also thank the beamline 07A1 of Taiwan Light Source (TLS) of the National Synchrotron Radiation Research Center (NSRRC) for X-ray absorption spectroscopy measurements. We express our gratitude for English editing software, such as Grammarly and ChatGPT, for refining language and checking grammatical errors in our manuscript. Open Access publishing facilitated by The University of Queensland, as part of the Wiley - The University of Queensland agreement via the Council of Australian University Librarians.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




