An Enzymatic Prodrug-like Route to Thio and Selenoamides
Abstract
6-Thioguanine (6TG) is a clinically used antitumor agent that was rationally designed as a DNA-targeting antimetabolite, but it also occurs naturally. 6TG is a critical virulence factor produced by Erwinia amylovorans, a notorious plant pathogen that causes fire blight of pome fruit trees. The biosynthesis of the rare thioamide metabolite involves an adenylating enzyme (YcfA) and a sulfur-mobilizing enzyme (YcfC), but the mechanism of sulfur transfer and putative intermediates have remained elusive. Through dissection and in vitro reconstitution of the thionation process using diverse substrates, we uncover an intermediate, prodrug-like thio-conjugate and elucidate the precise enzyme functions. YcfA not only adenylates GMP but also transfers the mercapto group of l-cysteine to the activated carbonyl. A designated C−S lyase (YcfC) then cleaves the resulting S-adduct to yield the thioamide. This pathway is distinct from canonical tRNA sulfur modifications and known enzymatic peptide thionations. By exploring a wide range of substrate surrogates, we exploited the tolerance of the enzyme pair to produce even a seleno analog. This study provides valuable insight into a previously unexplored area of bacterial thioamide formation and lays the groundwork for synthetic biology approaches to produce thioamide antimetabolites.
Introduction
Sulfur is an essential element for all life on Earth. It alters structural and chemical properties in important biomolecules such as amino acids and proteins, vitamins, co-factors, and tRNAs by forming disulfide bonds, thioesters, thioethers, and thioamides.1 The latter ubiquitously occur as tRNA modifications that warrant accurate codon recognition in ribosomal peptide biosynthesis.1b, 2 Moreover, thioamides occur as rare structural motifs in specialized metabolites and are critical for their biological activities. Important examples are the copper-chelating bacterial metabolites methanobactin3 and closthioamide,4 which is also an antibiotic that loses its potency when replacing the thioamide moieties with amides.5
Similarly, the antitumor activity of 6-thioguanine (6TG, 1) is based on the presence of the thioamide group, as the thio analogue functions as an antimetabolite that is incorporated into the target RNA and DNA of tumor cells. Since the early 1950s, 6TG has been used clinically, particularly for the treatment of chronic and acute leukemias, and is still listed (23rd list, 2023) by the WHO as an essential medicine.6 Although 6TG is best known as a synthetic drug conceived and developed by Nobel Prize winners Elion and Hitchings,7 it is also produced by plant-pathogenic bacteria, which apparently have been using this antimetabolite long before. We found that 6TG represents a critical virulence factor of Erwinia amylovora, the causative agent of fire blight.8 This plant disease causes one of the severest pome fruit symptoms and huge economic damages.9 By mutational analysis and heterologous expression we correlated 6TG biosynthesis to a small gene cluster (ycfA–D) that is widely distributed in genomes of pathogenic Erwinia species (Figure 1A).8a We also found that an enzyme pair, YcfA and YcfC, plays a central role in thioamide formation in guanosine phosphate precursors of 6TG.10 Initial bioinformatic analyses suggested that YcfA is an ATP-dependent sulfur transferase that is evolutionally related to tRNA modification enzymes.8a, 10
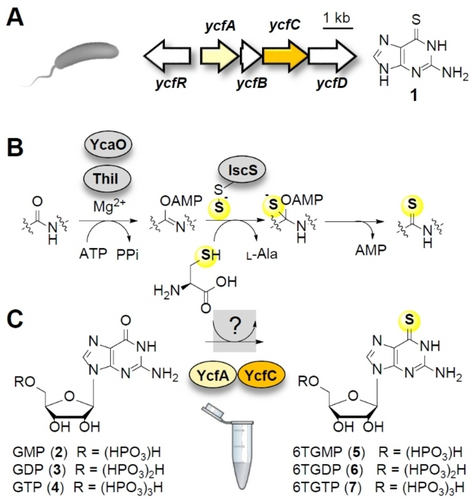
Model of thioamide formation in tRNA modification and specialized metabolism in bacteria. A) Structure of 6TG and architecture of the corresponding biosynthesis gene cluster (ycf) in E. amylovora. B) Canonical route for thioamidation of tRNA (ThiI) and peptides (YcaO). C) YcfA and YcfC are essential and sufficient for thioamidation of guanosine 5’-phosphates, yet mechanism and intermediates are unknown.
The formation of thioamides in tRNA nucleobases occurs through a process that involves the adenylation of the nucleobase (e.g. by ThiI), followed by the transfer of a persulfide sulfur onto the amide carbonyl oxygen (e.g. by IscS) (Figure 1B).1b Related thioamidation systems have been implicated for the biosynthetic pathways to closthioamide,11 thioviridamide,12 and thioholgamide.13 6TG biosynthesis, however, seems to deviate from this scheme, since YcfA requires a designated sulfur-mobilizing enzyme (YcfC) that differs from universal sulfur shuttles in RNA-related enzyme systems (Figure 1C).10 To date, the precise functions of the thioamidating enzyme pair, the mechanism of sulfur transfer from cysteine to guanine nucleobases, and potential biosynthetic intermediates have remained elusive.
Here we shed light on 6TG biosynthesis in E. amylovora and report a novel avenue for enzymatic thioamide formation. We reveal a previously unknown, prodrug-like 6TG intermediate, uncover the non-canonical function of an adenylation enzyme forming an amino acid thio-conjugate, and show that a designated C−S lyase cleaves this intermediate to yield 6-thioguanine nucleotides.
Results and Discussion
YcfC Functions as a C−S Lyase, but Not as a Desulfurase
The in vitro reconstitution of YcfA and YcfC shows that both enzymes play key roles in the thioamidation of GMP.10 The similarity of YcfA to thionizing RNA enzymes of the adenine nucleotide alpha-hydrolase-like (AANH-like) superfamily initially suggested that the sulfur is transferred in an analogous manner, namely by direct transfer of a reactive thiol generated by a Cys desulfurase.8a, 14 However, when testing YcfC in in vitro assays, we noted that it does not utilize inorganic sulfide as a source of sulfur.10
To gain insight into the mechanism of sulfur transfer and the true function of YcfC, we performed a phylogenetic analysis using amino acid sequences of YcfC and related enzymes from bacteria, fungi, and plants. The cladogram suggests that YcfC functions as a C−S lyase, but not as a Cys desulfurase (Figure 2A, Figure S1 and S2, Table S1). To confirm this, we conducted an in vitro assay with purified YcfC and l-Cys and analyzed the reaction products. Consistent with the phylogenetic prediction, we identified the expected outputs of a C−S lyase, namely pyruvate, ammonium, and hydrogen sulfide (Figure S3 and Tables S2–S4). It is important to note that l-Ala was not detected, which would have been formed by a desulfurase. Additionally, we mutated six cysteine residues of YcfC, which are crucial for a Cys desulfurase activity, but found that the mutants retained their function (Figure S4, Table S2–S4). These results suggest that YcfC does not function as a classical sulfur shuttle as is in known thioamide-forming enzyme systems but would cleave the C−S bond of an as yet unknown intermediate produced by YcfA.
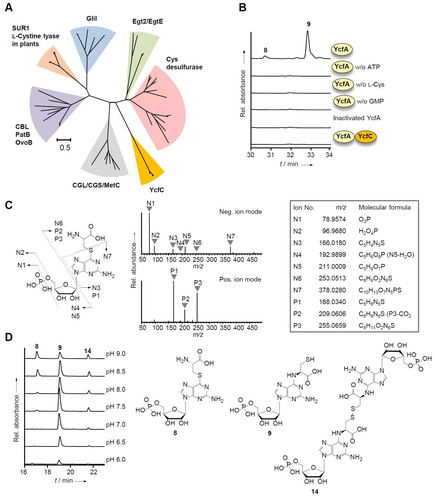
Discovery of an unstable intermediate produced by YcfA. A) Maximum likelihood phylogenetic tree of YcfC and C−S lyases. B) Reversed-phase ion pair HPLC profile of in vitro YcfA activity assay. C) The structure prediction of Cys-GMP S-adduct (8) by HR-ESI MS/MS analysis of 8. Left panel indicates proposed MS/MS fragmentation pattern of 8. Middle panel shows HR-ESI MS/MS (Neg. and Pos. ion modes) profiles of 8. Right panel shows detailed MS/MS profiles. D) Reversed-phase HPLC monitoring of pH dependent rearrangement of 8.
Discovery of an Unstable Intermediate Produced by YcfA
To scrutinize the unusual adenylation reaction and to detect elusive pathway intermediates, we reconstituted the YcfA-mediated reaction in vitro. First, we investigated a reaction mixture using only YcfA (in the absence of YcfC) and the minimally required additives, including ATP, Mg2+, l-Cys, DTT, and GMP at 30 °C for 22 h. By reverse-phase (RP) ion pair HPLC analysis of the reaction mixture we observed the formation of two new products with UV λmax at 285 nm (Figure 2B). Notably, these peaks were not observed in the absence of either ATP, l-Cys, or GMP (Figure 2B). However, we detected similar compounds when we replaced GMP with GDP or GTP (Figure S5). The RP-HPLC/HRMS analyses of the reaction mixtures containing GMP, GDP, or GTP revealed compounds (M−H)− with m/z 465.0661 (8 and 9), 545.0262 (10 and 11), and 624.9926 (12 and 13) (Figure S6, S9, S11). Their HR-MS/MS fragmentation pattern suggests that these compounds are l-Cys nucleotide adducts (Figures 2C, S10, S12). Time-course HPLC measurements and shifts in retention times indicated that these compounds are unstable. This is consistent with previous reports on purine S-adducts and unprotected aminothioesters that undergo intramolecular S,N-rearrangements.15 We observed a pH-dependency of the putative rearrangement and found that the primary l-Cys-GMP adduct is more stable under basic conditions (Figure 2D). HR-ESIMS2 analyses aided in distinguishing between the tentative l-Cys-GMP N-adduct (9) (and its dimer, 14) and the l-Cys-GMP S-adduct (8) (Figure S7, S8, S13, S14).
The Elusive Intermediate is an l-Cys-GMP S-Adduct
To rigorously confirm the structure of this intermediate, we aimed to isolate 8 formed in the in vitro reaction and to investigate it by NMR. Therefore, we added fully 13C and 15N labeled substrates, 13C3,15N-l-Cys and 13C10,15N5-GMP, to a large-scale enzyme assay. After many trials and repeated optimization of the reaction conditions and purification methods, we obtained a pure compound (Figure S15). However, based on the 13C NMR spectral analysis we inferred that this compound represents the l-Cys-GMP N-adduct (9) and not the S-adduct (Figure 3A and 3B, Figure S16–S19). By acetamide derivatization we confirmed that the compound has indeed a free mercapto group (Figure 3B, Figure S20–S22). Obviously, this compound has already undergone an S-N rearrangement during purification.
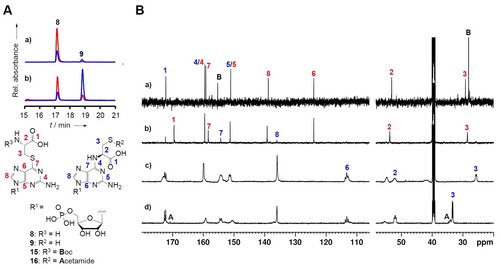
Structure elucidation of intermediate 8. A) Reversed-phase HPLC profile of a) synthetic standard and b) intermediates obtained by in vitro YcfA activity assay. Red line: 315 nm, blue line: 285 nm. B) 13C NMR comparison of four Cys-GMP congeners, a) synthetic Boc-l-Cys-GMP S-adduct (15), b) synthetic l-Cys-GMP S-adduct (8) with rearranged N-adduct (9), c) 13C- and 15N- labeled 9 obtained by in vitro YcfA assay, d) acetamide derivative (16) of 13C- and 15N-labeled 9.
To identify the primary reaction product, we synthesized an authentic reference, the l-Cys-GMP S-adduct (8), from 6-thioguanosine and protected l-β-iodoalanine (for details, see SI). We observed that, as the natural product, synthetic 8 undergoes a rapid S-N rearrangement into the N-adduct. Comparison of the 13C NMR spectra of the synthetic and natural N-adducts revealed their identical structures (Figure 3B, Figure S23–S30). Furthermore, by comparing the RP HPLC retention times of the synthetic S-adduct 8 and the product formed in the in vitro assay, we confirmed the identity of the intermediate and demonstrated that the S-adduct is the primary product of the YcfA-mediated biotransformation (Figure 3A). This finding not only contributes to the understanding 6-TG biosynthesis but also reveals a highly unusual function of an adenylating enzyme that uses GMP and an amino acid as substrates.
Substrate Specificity of YcfA
To learn more about this unexpected transfer of an amino acid onto the nucleotide base, we investigated the range of tolerated substrates. Since GMP, GDP and GTP are potentially accepted by the YcfA-YcfC enzyme pair, but not guanine or guanosine,10 we focused on a range of phosphorylated nucleosides. Specifically, we tested cyclic GMP (cGMP), deoxy GMP (dGMP), inosine monophosphate (IMP), uridine monophosphate (UMP), and xanthosine monophosphate (XMP) (Figure S32). By means of an adenylation activity assay based on the colorimetric quantification of released pyrophosphate,16 we found that YcfA has strongest preference towards GMP, followed by GDP and GTP. Although the adenylation assay suggested that YcfA could also accept cGMP and dGMP as substrates (Figure 4A), the corresponding thio adducts could not be detected by RP HPLC monitoring of the in vitro assays (Figure S33, Table S5).
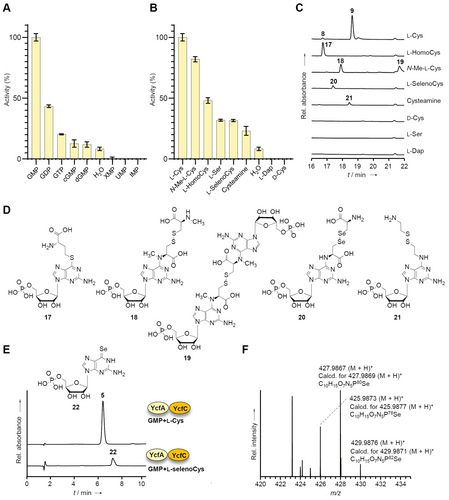
Determination of substrate specificity in YcfA. A) Adenylation activity assay of YcfA with l-Cys and diverse nucleotides. Activity obtained using GMP is used as 100 %. Error bar indicates SD. B) Adenylation activity assay of YcfA with GMP and diverse Cys congeners. Activity obtained using l-Cys is used as 100 %. Error bar indicates SD. C) Reversed-phase HPLC profiles of YcfA with GMP and diverse Cys congeners. D) Structures obtained from YcfA with GMP and diverse Cys congeners. E) Reversed-phase HPLC profile of in vitro production of 6TGMP (5) and 6-seleno-GMP (22). F) HR-ESIMS spectrum of 22.
It appears that in these cases the l-Cys thiol cannot attack the adenylated carbon due to an unfavorable positioning of adenylated GMP analogues in the substrate binding site. These results show that there is a degree of flexibility in the types of nucleobases, but YcfA clearly prefers GMP as a substrate (Figure 4A).
Next, we explored the scope of potential cosubstrates and tested various compounds as surrogates of l-Cys, including d-Cys, N-methyl-l-Cys (N-Me-l-Cys), l-homocysteine, l-selenocysteine (from l-selenocystine), l-Ser, l-Dap (l-2,3-diaminopropanoic acid), and cysteamine. The in vitro assay revealed that YcfA has a clear preference for l-Cys, but it can also accept numerous analogues, except for l-Dap and d-Cys (Figure 4B, Table S5). To corroborate these results, we also monitored the assays by RP-HPLC (Figure 4C) and LC-HRMS2 (Figure S34–S43). We detected the masses of the expected S- or N-adducts of GMP with N-methyl-l-Cys, l-homocysteine, l-selenocysteine, and cysteamine (17–21) (Figure 4C, D). The different tendency to form S-adducts (17), N-adduct homodimers (19), and N-adduct heterodimers (18, 20, 21) of GMP likely depends on the formation to five- or six-membered cyclic intermediates and the rate of the rearrangement.
The pyrophosphate release assay revealed that YcfA accepts a range of thio and even seleno nucleophiles but is unable to use amino (l-Dap) or hydroxy (l-Ser) analogues. GMP is activated by ATP in the presence of Ser, yet the amino acid cannot be loaded. In contrast, all tested l-Cys surrogates that harbor thiol groups are accepted and loaded onto GMP. The most remarkable finding is that even the selenol analogue is converted into the corresponding adduct. We also observed the formation of 6-seleno GMP (22), the selenium isologue of 6TGMP (5), in the YcfA-YcfC assay (Figure 4E, F, Figure S44–S46).
YcfC is a Designated Thioamide-Forming C−S lyase
After having established the first part of the thionation reaction, we focused on analyzing the subsequent biotransformation that generates the thioamide moiety through scission of the thioether adduct. To achieve this, we reconstituted and optimized the enzymatic C-S bond cleavage in vitro using recombinant lyase YcfC, synthetic l-Cys-GMP S-adduct (8), DTT, and PLP, at pH 8.5 (Figure S47) and 30 °C. We monitored the successful production of 6-thio-GMP by RP-HPLC using an authentic reference (Figure S64). Additionally, we detected pyruvate as a side product through derivatization with 2,4-dinitrophenylhydrazine (DNPH), followed by colorimetric detection (Figure 5) and RP-HPLC analysis (Figure S48). These results unequivocally demonstrate that YcfC is a C−S lyase.
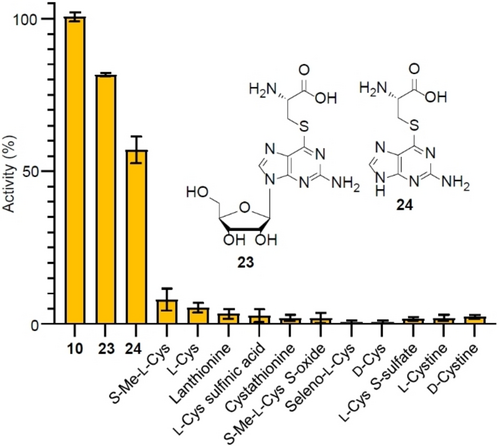
C−S lyase assay based on pyruvate detection using DNPH. Activity obtained using L-Cys GMP S-adduct is used as 100 %. Error bars indicate SD.
To investigate the substrate specificity of YcfC, we tested synthetic l-Cys S-adducts of guanosine (23) and guanine (24) (Figure S49–S56). Additionally, we probed eleven GMP S-adducts with Cys surrogates, including l-Cys, d-Cys, l-cystine, d-cystine, l-selenocysteine (released by reducing l-selenocystine), l-cystathionine, l-lanthionine, S-methyl-l-Cys sulfoxide, S-methyl-l-Cys, l-Cys sulfinic acid, and l-Cys S-sulfate (Figure 5, Figure S57). This experiment showed that YcfC has a clear preference for the S-adducts 8, 23, and 24. It also indicates that YcfC requires at least a guanine moiety in l-Cys S-adducts.
We performed kinetic analyses of YcfC using synthetic substrates 8, 23, and 24, which are among the top three candidates accepted by YcfC, under optimized assay conditions (Figure 5). To determine Km and kcat values we measured the increase of absorbance at 340 nm, which is diagnostic (λmax=340 nm) for the resulting 6-thio compounds. We determined the Km and kcat values for substrate 8 to be Km=50.4±5.0 μM and kcat=2.1±0.1 s−1, for compound 23, Km=359.1±24.8 μM and kcat=3.9±0.2 s−1, and for compound 24, Km=317.2±23.6 μM and kcat=2.7±0.1 s−1 (Figures S58–S64). These results show that YcfC has an apparent affinity to 8, while the turnover number is similar for all three substrates.
A phylogenetic analysis of YcfC and other β-lyases indicates that YcfC falls into a new clade (Figure 2A). Interestingly, however, an E. amylovora mutant lacking the ycfC still produces substantial amounts of 6TG (83 % of the wild-type strain).8a This observation indicates that another C−S lyase is capable of cleaving the l-Cys-GMP S-adduct instead of YcfC. To confirm this and to further investigate the specificity of the enzymatic C−S cleavage, we tested the ability of lyases that represent other clades and employ different substrates, to cleave the l-Cys-GMP S-adduct (8). Specifically, we tested another E. amylovora lyase, OvoB from bacterial ovothiol A biosynthesis,17 and other lyases, Egt2 from fungal ergothioneine biosynthesis,18 SUR1 from glucosinolate biosynthesis in plants,19 and the cystathionine β-lyase MetC.20 The corresponding genes (ovoB, egt2, sur1, and metC) were individually subcloned into an E. coli expression vector (pET28a, Figures S65 and S66), and the heterologously produced His6-tagged lyases were purified the Ni-affinity columns (Figures S67 and S68). When we substituted YcfC with these alternative lyases in the standardized YcfA/YcfC assay, we were still able to detect 6TGMP production (Figure S69 and S70). This result clarified not only YcfC, but also OvoB and diverse lyases could cleave C−S bond of 8. However, when we compared lyase activities with synthetic thio-conjugate 8, we found that only YcfC readily cleaves the thioether bond, whereas the activity of the other lyases is negligible (Figures S71–S73). This finding indicates that YcfC is a designated lyase for thioamide formation.
Conclusion
Enzymatic C−S and C−Se bond formations have become a significant area of interest for synthetic biology and chemo-enzymatic synthesis, as they present mild and environmentally friendly alternatives to the harsh conditions and toxic reagents typically employed in organic synthesis. This is particularly true for thioamides, which are valued for their versatile properties as chelators and pharmacophores, but whose chemical synthesis from amides requires high temperatures and harmful phosphorus reagents (e.g. Lawesson's).21
This study sheds light on a novel mechanism of thioamide formation, a key step in the biosynthesis of the antimetabolite 6-thioguanine. Our in vitro studies revealed that YcfA is an unusual adenylating enzyme that not only activates the amide carbonyl of GMP using ATP, but also transfers l-cysteine to form a thio-conjugate. This process is intriguing as it markedly deviates from the well-studied adenylating enzymes that activate amino acids in non-ribosomal peptide synthetases (Figure S74).22
Another peculiar finding is that thioamide formation occurs through cleavage of the S-adduct by a specialized C−S lyase, YcfC. This pathway differs significantly from known routes to thioamides involving adenylate-forming enzymes that use persulfides provided by cysteine desulfurases, as in tRNA modification14 and in the biosynthesis of thioamidated peptides,11-13, 23 and from the ATP-independent thioamidation in methanobactin biosynthesis.24 While lyase-mediated C−S cleavage reactions of thioethers (and sulfoxides) have been observed in a number of biosynthetic pathways, e.g. to gliotoxin,25 ergothioneine,18 and ovothiol,17c the route to a thioamide is novel (Figure S1 and S75).
The liberation of a 6-thioguanine precursor by C−S bond cleavage is highly intriguing because it mirrors the prodrug strategies used in medicinal chemistry to reduce the side effects of 6TG and related antimetabolites for their use in chemotherapy.26 A famous, and surprisingly similar, example is S-(guanin-6-yl)-l-cysteine (24), a selective 6TG prodrug that is activated by renal C−S β-lyases (Figure 6).27
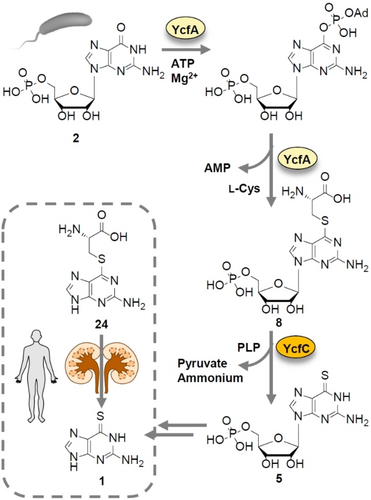
Model of thioamide formation in E. amylovora and its analogy to a 6TG prodrug system.
Notably, the unique YcfA/YcfC-mediated biotransformation can also be extended to form C−Se bonds. As such, our finding is an important addition to the very few selenium-incorporating biocatalysts from specialized metabolism, namely a fungal sulfoxide synthase-lyase system28 and a selenosugar synthase-glycosyltransferase system,29 both of which lead to selenoneine,30 the selenium analogue of ergothioneine. Very recently chemoenzymatic seleno compound synthesis by using selenium carrier proteins has been also established.31
Finally, our findings are also relevant from an agricultural perspective. 6TG is a critical virulence factor of the notorious plant pathogen E. amylovora, which causes devastating losses in pome tree plantations, and due to antibiotic resistance it is very difficult to control fire blight with currently available means.9b, 32 Since the presence of 6TG is crucial for the pathogen to cause the torched-looking, dead leaves, a detailed knowledge of the enzymatic transformations may inspire the development of specific inhibitors for antivirulence strategies. Thus, in addition to uncovering an unusual, new route to thio and selenoamides, which has prospects for chemoenzymatic and synthetic biology applications, our findings have potential translational value for controlling severe plant disease outbreaks.
Supporting Information
The authors have cited additional references within the Supporting Information.
Acknowledgments
We thank A. Perner, F. Trottmann, and B. Bartels for LC-HRMS measurements. We also thank H. Heinecke for NMR measurements. Financial support by the Humboldt foundation for a postdoctoral fellowship (to K. D.), by the German Science Foundation (DFG, Leibniz prize to C. H.), and by the European Regional Development Fund (ERDF) (MassNat) is gratefully acknowledged. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.




