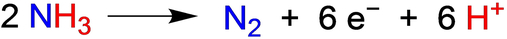Oxidation of Ammonia Catalyzed by a Molecular Iron Complex: Translating Chemical Catalysis to Mediated Electrocatalysis
Abstract
Ammonia is a promising candidate in the quest for sustainable, clean energy. With its capacity to serve as an energy carrier, the oxidation of ammonia opens avenues for carbon-neutral approaches to address worldwide growing energy needs. We report the catalytic chemical oxidation of ammonia by an Earth-abundant transition metal complex, trans-[LFeII(MeCN)2][PF6]2, where L is a macrocyclic ligand bearing four N-heterocyclic carbene (NHC) donors. Using triarylaminium radical cations in MeCN, up to 182 turnovers of N2 per Fe were obtained from chemical catalysis with an extremely low loading of the Fe catalyst (0.043 mM, 0.004 mol % catalyst). This chemical catalysis was successfully transitioned to mediated electrocatalysis for the oxidation of ammonia. Molecular electrocatalysis by the Fe catalyst and the mediator (p-MeOC6H4)3N exhibited a catalytic half-wave potential (Ecat/2) of 0.18 V vs [Cp2Fe]+/0 in MeCN, and achieved 9.3 turnovers of N2 at an applied potential of 0.20 V vs [Cp2Fe]+/0 at −20 °C in controlled-potential electrolysis, with a Faradaic efficiency of 75 %. Based on computational results, the catalyst undergoes sequential oxidation and deprotonation steps to form [LFeIV(NH2)2]2+, and thereafter bimetallic coupling to form an N−N bond.
Introduction
Achieving a fundamental, molecular-level understanding of the oxidation of NH3 to generate benign N2 requires exquisite control of the six-proton, six-electron oxidation process (eq. 1).13 In contrast to well-documented multiple-electron, multiple-proton processes, such as oxidation of water to O2, oxidation of ammonia to N2 has been much less explored. NH3 generally exhibits excellent coordination ability as a σ donor, facilitating coordination-induced N−H activation, thereby either weakening the N−H bond for homolytic hydrogen atom transfer (HAT)14-25 or increasing the acidity of the N−H bond for deprotonation.20-22, 26 Proton-coupled electron transfer (PCET) processes of one-electron oxidation and deprotonation emerge as plausible routes for the catalytic ammonia oxidation, attainable through either electrochemical methods27-38 or chemical oxidizing agents28, 32 in the presence of suitable bases. Alternatively, homolytic cleavage of N−H bonds can be achieved through HAT using aryloxyl radicals in the context of chemical catalysis for ammonia oxidation.39-41
Ruthenium complexes have a particularly prominent and successful role in recently reported molecular catalysts for oxidation of ammonia.27, 28, 31, 33, 36, 37, 39, 40, 42, 43 Efforts to improve sustainability have focused on the design of catalysts based on Earth-abundant metals.44 Peters and co-workers reported an Fe electrocatalyst that has two cis-labile coordinating sites, [(TPA)FeII(MeCN)2]2+ (TPA=tris(2-pyridylmethyl)amine), for electrochemical oxidation of NH3.29 Sixteen turnovers were achieved for N2 production, with an onset potential of about 0.7 V vs [Cp2Fe]+/0. Having observed that catalyst degradation was likely caused by competitive binding of NH3 to high-spin [(TPA)FeII(NH3)2]2+, Peters and co-workers designed a more robust low-spin FeII electrocatalyst, [(bpyPy2Me)FeII(MeCN)2]2+, which displayed 149 turnovers of N2 with an onset potential of 0.45 V.30 Very recently, Peters and co-workers further improved the Fe catalyst by installing two MeO substituents on the pyridine moieties of the bpyPy2Me ligand. Their new catalyst achieved 381 turnovers of N2 at a lower onset potential of 0.29 V, with essentially 100 % Faradaic efficiency.38 Nishibayashi, Sakata, and co-workers unveiled a MnII salen catalyst for catalytic oxidation of ammonia.32 Chemical catalysis using [(p-BrC6H4)3N]⋅+[SbCl6]− (“Magic Blue”) as the oxidizing agent gave up to 17 turnovers of N2, while electrocatalysis furnished 6.6 turnovers of N2 with a Faradaic efficiency of 96 % at an onset potential of 0.66 V. Wang, Liao, Ye, and co-workers discovered an FeIII complex, [Cp*FeIII(1,2-Ph2PC6H4NH)(NH3)]+ (Cp*=C5Me5), supported by a non-innocent ligand, capable of catalyzing ammonia oxidation with an onset potential of −0.04 V, yielding 4.4 turnovers of N2.34 Warren and co-workers discovered chemical and electrochemical oxidation of NH3 by ferrocenium to give N2.45
Moving across the periodic table beyond iron, Mock and co-workers discovered a NiII complex, [CpNiII(IMes)(NH3)]+ (Cp=C5H5; IMes=1,3-bis(2,4,6-trimethylphenyl)-1,3-dihydro-2H-imidazol-2-ylidene), that catalyzed chemical oxidation of NH3 using organic radicals to abstract hydrogen atoms,41 giving up to 56 turnovers of N2. Warren and co-workers reported electrochemical oxidation of ammonia with an onset potential of −0.24 V with 18 turnovers of N2, using a tri-coordinate neutral CuI complex supported by a β-diketiminato ligand.35 In addition, Brudvig and co-workers found a CuI catalyst [Cu(bipyalk)]+ [bipyalkH=2-[(2,2′-bipyridin)-6-yl]propan-2-ol] that catalyzes the oxidation of NH3 in aqueous solutions to nitrite and nitrate, achieving a 94 % combined Faradaic efficiency without interference from water oxidation.46
Redox mediators can significantly improve organic electrosynthesis47-49 and electrocatalytic reactions involving fuel cell half-reactions, such as O2 reduction50, 51 and oxidation of alcohols.49, 52-55 Molecular mediators offer striking advantages in electrocatalysis, primarily by facilitating heterogeneous electron transfer between the electrode and homogeneous solutions and/or circumventing high-energy reaction intermediates. Consequently, mediated electrocatalysis provides an appealing approach to develop electrocatalysts capable of achieving fast reaction rates at low overpotentials (high energy efficiency). Despite recent advances, electrocatalytic ammonia oxidation enhanced by organic redox mediators has not yet been reported, to the best of our knowledge. Furthermore, substantial improvements remain imperative to reduce the overpotentials of electrocatalysts and improve catalyst lifetimes.
Herein, we report the catalytic oxidation of ammonia using an Earth-abundant iron complex bearing a macrocyclic ligand with four N-heterocyclic carbene (NHC) donors. This Fe complex functions as both a chemical catalyst and as an electrocatalyst mediated by triarylamine for oxidation of NH3 at low temperatures. In the realm of chemical catalysis, this Fe-based catalyst displayed remarkable efficiency, yielding up to 182 turnovers of N2 at −30 °C using a mild oxidizing agent, [(p-MeOC6H4)3N]⋅+, when operating close to the liquefaction point of ammonia (−33 °C). This exceptional catalytic performance was realized at an unusually low loading of the Fe catalyst, approximately 0.043 mM (0.004 mol %). Synergistic electrocatalysis promoted by a molecular catalyst and an organic mediator was developed for oxidation of ammonia for the first time. Electrocatalysis by the Fe catalyst and [(p-MeOC6H4)3N] exhibited an onset potential of 0.09 V and an Ecat/2 of 0.18 V vs [Cp2Fe]+/0, and achieved 9.3 turnovers of N2 at an applied potential of 0.20 V vs [Cp2Fe]+/0 with a Faradaic efficiency of 75 % for electrocatalysis carried out at −20 °C. In mediated electrocatalysis, [(p-MeOC6H4)3N]⋅+ likely facilitates heterogeneous electron transfer process from the electrode to solutions as well as PCET reactions occurring at axial NxHy moieties. Preliminary density functional theory (DFT) calculations suggest that N−N bond formation occurs via the formation of an [LFeIV(NH2)2]2+ intermediate, followed by bimetallic coupling and formation of a hydrazido complex.
Results and Discussion
NH3 Binding to FeII in MeCN
Catalyst degradation reported in previous studies of ammonia oxidation was proposed to occur from either competitive binding of NH3 to the metal center29, 38 or protonation of an anionic ligand by [NH4+].35 Considering these limitations, we explored the use of an iron complex, trans-[LFeII(MeCN)2][PF6]2, bearing a macrocyclic ligand (abbreviated hereafter as L) with four N-heterocyclic carbene (NHC) donors. The strong-field tetradentate macrocyclic ligand is expected to confer enhanced stability. In addition, the Fe center in the cyclic tetra-NHC environment is electron-rich because of the strongly σ-donating NHC ligand, facilitating the first oxidation step in catalytic ammonia oxidation. Trans-[LFeII(MeCN)2][PF6]2 was first reported by Kühn and co-workers,56 who showed that the axial MeCN ligands of trans configurations can be replaced by DMSO, NO, CO, tBuNC or Ph3P.56-58 The reaction of [LFeII(MeCN)2]2+ with O2 and catalytic epoxidation of alkenes have also been reported.57, 59-61 Notably, NH3 binding to an acyclic tetra-NHC FeII complex with cis-labile binding sites was recently reported by our group.62
Addition of 15NH3 gas (1.2 atm) to a light yellow solution of [LFeII(MeCN)2]2+ in CD3CN at 22 °C produced a deepened yellow solution that contained approximately 2.8 M of 15NH3. The 1H NMR spectra revealed a mixture of [LFeII(MeCN)2]2+, [LFeII(NH3)(MeCN)]2+ and [LFeII(NH3)2]2+ (Scheme 1) in a molar ratio of 5 : 63 : 32. All the 1H NMR spectroscopic signals were observed in the normal range of chemical shifts, indicating diamagnetic low-spin FeII species. Free 15NH3 was observed at 0.52 ppm in the 1H NMR spectrum, compared to 0.45 ppm for 15NH3 in CD3CN at 1.0 atm with no FeII complex added, suggesting that the bound 15NH3 ligands exchange with free 15NH3. In addition to the 1H NMR spectroscopic signals of [LFeII(MeCN)2]2+, two sets of new resonances assigned to 15NH3-bound FeII complexes were observed (Figure S11). Doublets for bound 15NH3 at −0.93 ppm (1J15N−1H=67.5 Hz) and −1.49 ppm (1J15N−1H=67.2 Hz) are assigned to [LFeII(15NH3)(MeCN)]2+ and [LFeII(15NH3)2]2+, respectively. In the 15N NMR spectrum, two quartets for bound 15NH3 ligands were observed at −442.5 (1J15N−1H=67.3 Hz) and −450.5 (1J15N−1H=66.8 Hz) ppm, both of which are upfield from free 15NH3 (−370.0 ppm; Figure S19). The observed doublets in the 1H NMR spectrum and quartets in the 15N NMR spectrum for bound 15NH3 ligands all collapsed into singlets in the 1H{15N} and 15N{1H} NMR spectra (Figures S15 and S20).
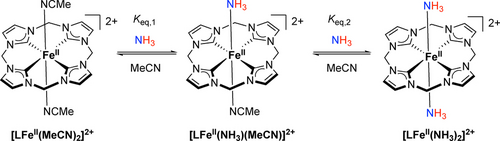
Replacement of axial MeCN ligands on FeII by NH3.
The assignment was further supported by 1H NMR experiments on [LFeII(MeCN)2]2+ with incrementally increasing concentrations of NH3, in which the 1H signals of [LFeII(NH3)(MeCN)]2+ emerged first, followed by those of [LFeII(NH3)2]2+(Figure S25). Upon increasing [NH3] from 0.012 to 0.27 M, the signals of bound NH3 for both [LFeII(NH3)(MeCN)]2+ and [LFeII(NH3)2]2+ shifted slightly towards that of free NH3, moving downfield from −1.13 to −1.11 ppm for the former and from −1.66 to −1.64 ppm for the latter, again indicative of dynamic binding equilibria of NH3 to the FeII complexes.
At [NH3] <0.051 M, only the equilibrium between [LFeII(MeCN)2]2+ and [LFeII(NH3)(MeCN)]2+ was observed, with no [LFeII(NH3)2]2+ detected. At higher NH3 concentrations (0.10–2.8 M), [LFeII(NH3)2]2+ was observed, leading to two interlinked equilibria. The first equilibrium constant (Keq,1 in Scheme 1) was determined by eq. S2 in Scheme S3 using the equilibrium concentrations of NH3, [LFeII(MeCN)2]2+, and [LFeII(NH3)(MeCN)]2+. The second equilibrium constant (Keq,2) was determined through two distinct methods. The first method paralleled the approach employed for Keq,1, relying on the equilibrium concentrations of NH3, [LFeII(NH3)(MeCN)]2+, and [LFeII(NH3)2]2+ (eq. S4 in Scheme S4). The second method was derived based on the concept of a defined fractional saturation, which was originally developed for binding of O2 to hemoglobin (eq. S5 in Scheme S5, details in the Supporting Information).63, 64 The equilibrium constants for binding of NH3 at 298 K were determined as Keq,1≈3.6 M−1 and Keq,2≈0.17 M−1. As estimated using the obtained Keq,1 and Keq,2, the population of [LFeII(NH3)(MeCN)]2+ is approximately 69 %, whereas [LFeII(MeCN)2]2+and [LFeII(NH3)2]2+ constitute about 19 % and 12 %, respectively, at 298 K and 1.0 M NH3 in MeCN.
A crystal of [LFeII(NH3)(MeCN)][PF6]2 was grown by vapor diffusion of Et2O to a MeCN solution in the presence of concentrated NH3 at −30 °C, and the structure was determined by single crystal X-ray diffraction (Figure 1).65 The flexibility of four CH2 linkers allows the ligand to adopt a distorted saddle conformation, while the FeII maintains a slightly distorted octahedral geometry, similar to that found for [LFeII(MeCN)2]2+.56 The FeII−NH3 bond length of 2.0603(16) Å is in the typical range of low-spin NH3-bound FeII complexes (2.03–2.08 Å).30, 62, 66-68
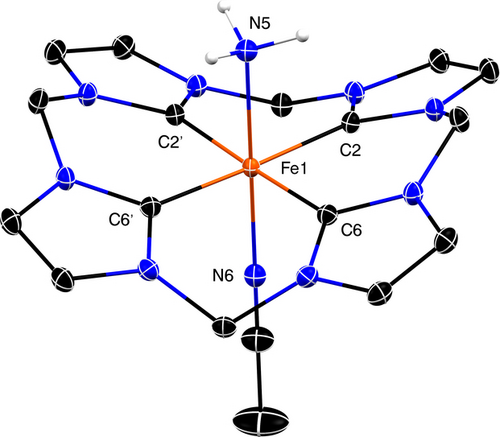
Molecular structure of [LFeII(NH3)(MeCN)][PF6]2, with thermal ellipsoids at the 30 % probability level. [PF6]− anions and hydrogen atoms (except NH3) are not shown.
NH3 binding to FeIII in MeCN.
The FeIII complex, [LFeIII(MeCN)2]3+, was prepared by one-electron oxidation of [LFeII(MeCN)2]2+ (E1/2=0.15 V in MeCN, all reduction potentials are referenced to [Cp2Fe]+/0) by thianthrene radical cation (E1/2=0.86 V in MeCN).59 A deep purple solution of [LFeIII(MeCN)2]3+ was treated with 2.5 equiv. of NH3 in MeCN at −30 °C, resulting in an immediate color change to yellow (Scheme 2). After recrystallization by layering Et2O onto the MeCN solution, [LFeIII(NH3)2]3+ was isolated as a yellow solid in ∼61 % yield. Note that excess NH3 would reduce FeIII to FeII (see details below).
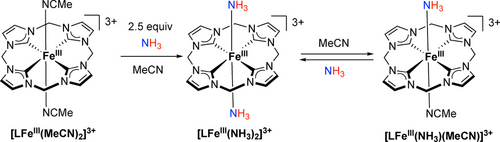
Binding and dissociation of NH3 ligands on FeIII.
In the 1H NMR spectrum of the paramagnetic FeIII complex in CD3CN, two distinct singlets with equal integrals (8H) were observed at −16.8 and 39.4 ppm, assigned to CH and CH2, respectively, along with a broad resonance for bound NH3 at 257.1 ppm (Figure S27). The assignment of bound NH3 was confirmed by its almost complete absence in the 1H NMR spectrum of [LFeIII(ND3)2]2+ (99 atom % D) (Figure S31), and was further verified by a sharp signal at 256.6 ppm in the 2H NMR spectrum in CD3CN (Figure S32).
Slow dissociation of the axial NH3 ligands of [LFeIII(NH3)2]3+ was observed, as indicated by the conversion of 25 % of [LFeIII(NH3)2]3+ after 3 days of vacuum drying. This drying process followed by dissolution in CD3CN generated [LFeIII(NH3)(CD3CN)]3+, which exhibited 1H NMR signals at −16.0 ppm for CH, 39.1 and 45.5 ppm for its diastereotopic CH2, and 252.7 ppm for the bound NH3 (Figures S33 and S34). Similarly, the replacement of NH3 by the solvent CD3CN becomes more pronounced as the concentration of [LFeIII(NH3)2]3+ decreases, as indicated by a concomitant increase in the amount of [LFeIII(NH3)(CD3CN)]3+ from 5 % in an 8 mM solution to 21 % in a 0.8 mM solution (Figure S35). These observations imply stronger binding of FeIII−NH3 compared to FeII−NH3 (see below), as expected, since FeIII is more electron deficient than FeII and behaves as a harder Lewis acid towards σ-donor ligands like NH3.
A solution of [LFeIII(NH3)2]3+ in MeCN (∼55 μM Fe), which includes both [LFeIII(NH3)2]3+ and [LFeIII(NH3)(MeCN)]3+ upon dissolution, displays a broad adsorption band at 450 nm (ϵ=1944 M−1 cm−1) in the UV/Vis spectrum, which shifts from 500 nm (ϵ=2202 M−1 cm−1) and 570 nm (ϵ=2197 M−1 cm−1) for [LFeIII(MeCN)2]3+, consistent with the striking color change from deep purple to bright yellow (Figure S29). [LFeIII(NH3)2]3+ is likely a low-spin FeIII complex (S= ), as determined by the Evans method (see details in the Supporting Information).69 [LFeIII(NH3)2]3+ displays N−H stretching frequencies at 3322, 3297 and 3231 cm−1 in the solid-state infrared spectrum (3337, 3450 cm−1 for gaseous NH3),70 compared to the N−D bands at 2481, 2386 and 2345 cm−1 for [LFeIII(ND3)2]3+. The ratios of N−D vs N−H stretching frequencies (0.75, 0.72, and 0.73) are consistent with the value of 0.73 calculated from Hooke's law.
The molecular structure of [LFeIII(NH3)2]3+ was determined by single-crystal X-ray diffraction on a crystal grown by vapor diffusion of Et2O into a MeCN solution at −30 °C. (Figure 2). The conformation of the supporting ligand and the coordination geometry of Fe in [LFeIII(NH3)2]3+ are similar to those of [LFeIII(MeCN)2]3+[59] and [LFeII(NH3)(NCMe)]2+; all the three complexes exhibit two axial ligands in a trans configuration. Notably, the FeIII−NH3 bond (1.964(8) Å) in [LFeIII(NH3)2]3+ is shorter than those of previously reported FeIII complexes with NH3 ligands (1.994–2.080 Å), which all bear more electron-rich di- or tri-anionic supporting ligands.20, 34, 71-73
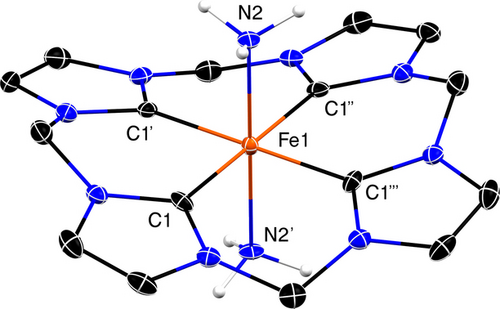
Molecular structure of [LFeIII(NH3)2][PF6]3 with thermal ellipsoid plot drawn at the 30 % probability level. [PF6]− anions and hydrogen atoms (except NH3) are not shown.
Electrochemical Studies
The slow replacement of axial NH3 by MeCN upon dissolution allows an investigation of the electrochemical properties of [LFeIII(NH3)2]3+ (bottom left in Scheme 3). Three redox events were observed using differential pulse voltammetry (DPV, also used for differential pulse voltammogram) and cyclic voltammetry (CV, also used for cyclic voltammogram). In Figure 3, E1, E2 and E3 represent the E1/2 values, based on DPV, while Ez,pc and Ez,pa (z=1, 2, 3) represent the peak potential of cathodic and anodic events in the CV, respectively. In Scheme 3, electrochemical reductions and oxidations are denoted as Ez,red and Ez,ox (z=1, 2, 3). As shown in the DPV, a redox wave at 0.15 V (E1,red) is assigned to the reduction of [LFeIII(MeCN)2]3+, in agreement with the previously reported redox potential of FeIII/II at 0.15 V for [LFeII(MeCN)2]2+.56 The reduction wave at −0.15 V (E2,red) likely results from [LFeIII(NH3)(MeCN)]3+. The observed peak current corresponds to 25 % [LFeIII(NH3)(MeCN)]3+ in a 0.8 mM solution, which agrees with the result obtained from the 1H NMR experiment above where 21 % [LFeIII(NH3)(MeCN)]3+ was detected in a solution of the same concentration. The major redox event at −0.44 V (E3,red) is assigned to the reduction of [LFeIII(NH3)2]3+.
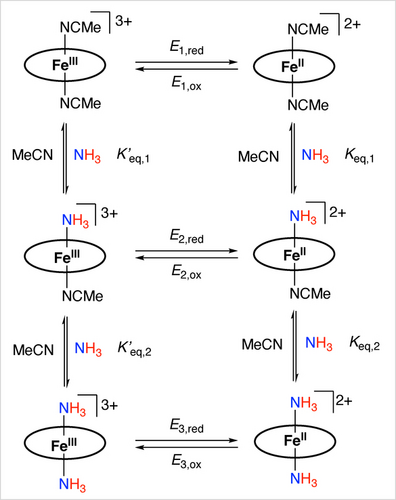
Replacement of axial ligands and redox transformations of [LFeII(MeCN)2-x(NH3)x]2+ and [LFeIII(MeCN)2-x(NH3)x]3+ (x=0, 1, 2). The ellipse represents the macrocyclic tetra-carbene ligand.
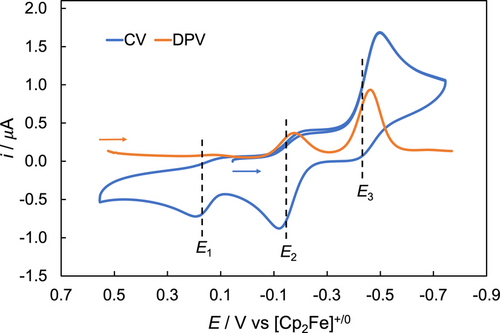
CV (blue) and DPV (orange) of 0.8 mM [LFeIII(NH3)2]3+ in MeCN under 1 atm N2 recorded in 0.1 M [n-Bu4N][B(C6F5)4] supporting electrolyte. [Cp2Co][PF6] was added as the internal standard, but is not shown in this window. Scan rate=100 mV/s for the CV. Ep=E1/2–ΔEpulse/2 for DPV,74 with ΔEpulse representing the pulse amplitude (50 mV in our experiments).
In the CV, the reduction of [LFeIII(NH3)2]3+ (E3,pc=−0.50 V) on the cathodic scan leads to replacement of the axial NH3 ligands by the solvent MeCN at a scan rate of 0.1 V/s, resulting in a very small anodic peak at E3,pa=−0.42 V for [LFeII(NH3)2]2+ along with increased anodic peaks at E2,pa=−0.12 V for [LFeII(NH3)(MeCN)]2+ and E1,pa=0.20 V for [LFeII(MeCN)2]2+ on the return scan. The observation agrees with the favorable formation of [LFeII(NH3)(MeCN)]2+ and [LFeII(MeCN)2]2+ over [LFeII(NH3)2]2+ in the Fe(II) oxidation state. When the scan rate was increased to 10 V/s, a quasi-reversible redox event of E3 with ipa/ipc=0.74 and ΔEp=187 mV (compared to ΔEp=82 mV for [Cp2Fe]+/0) was observed, allowing determination of the FeIII/II redox potential of [LFeIII(NH3)2]3+ as approximately −0.47 V (Figure S52).
As described above, upon replacement of each MeCN in [LFeII(MeCN)2]2+ by NH3, the redox potential of FeIII/II shifts cathodically by approximately 300 mV, and substitution of two axial MeCN ligands by NH3 ligands results in a cumulative cathodic shift of about 600 mV. Similar trends have been previously reported. Upon replacing two MeCN ligands by NH3, a cathodic shift of ∼300 mV for [(TPA)FeII(MeCN)2]2+, 460 mV for [NMe2(NCCN)Fe(MeCN)2]2+, and 680 mV for [acyclic-(NHC)4Fe(MeCN)2]2+ was observed, respectively.29, 62
in which Keq=Keq,1Keq,2=0.62 M−2 and ΔE°=E3–E1 (more details are shown in Scheme S13).
Based on this approach, Keq' was estimated to be 2.4×1010 M−2, which is ten orders of magnitude greater than Keq, providing quantitative evidence on the superior binding affinity of NH3 to FeIII compared to FeII.
Chemical Catalysis of Ammonia Oxidation
We previously discovered that oxidation of NH3 to N2 is catalyzed by RuII(TMP)(NH3)2 (TMP=tetramesitylporphyrin) when treated with an aryloxyl radical that abstracts hydrogen atoms.40 In contrast, no N2 was detected by 15N NMR spectroscopy when a solution of [LFeII(MeCN)2]2+ and 15NH3 in CD3CN was treated with either TEMPO (bond dissociation free energy (BDFE) of TEMPO−H=66 kcal/mol)75 or tri-tert-butyl aryloxyl radical (BDFE of ArO−H=74.8 kcal/mol).75
We directed our focus to the removal of electrons by a one-electron chemical oxidant and deprotonation by an organic base. This approach inherently presents the challenge arising from the commonly observed poor compatibility between oxidizing agents and electron-rich bases.76 Pioneering work by Nishibayashi, Sakata, and co-workers addressed the chemical compatibility issue by conducting the oxidations at low temperatures.28, 32 They carried out catalytic oxidations of NH3 using [(p-BrC6H4)3N]⋅+[SbCl6]− (E1/2=0.67 V in MeCN)77 as the oxidant and 2,4,6-trimethylpyridine (collidine) as the base. Note that the basicity of 2,4,6-trimethylpyridine is weaker than that of NH3 in MeCN (pKa=15.0 for 2,4,6-trimethylpyridinium; pKa=16.46 for [NH4+]).78, 79 Consequently, only a small fraction of free NH3 would be generated from 1 : 1 [NH4][OTf]/collidine (Figure S42 and Table S11, ∼9 % at room temperature, though it may vary at lower temperatures). While NH3 is being consumed during the catalysis, additional NH3 would be continually produced in the presence of [NH4][OTf] and collidine, thereby possibly maintaining a constant, low concentration of NH3. Additionally, we envision that using bases stronger than NH3 in the catalytic reaction can lead to other complications, since strong bases are more easily oxidized. Our experiments utilized free NH3 in solution for the chemical catalysis, in which NH3 serves both as the substrate to be oxidized and as the base for deprotonation.27-37
The speciation of [LFeII(MeCN)2-x(NH3)x]2+ (x=0, 1, 2) displayed redox potentials of FeIII/II lower than about 0.15 V vs [Cp2Fe]+/0 (see details in Figures 3 and 4). The oxidizing agent [(p-MeOC6H4)3N]⋅+[PF6]− (E1/2=0.16 V in MeCN)80 was selected for chemical catalysis. Treatment of [LFeII(MeCN)2]2+ with 24 equiv. of deep blue [(p-MeOC6H4)3N]⋅+ in an NMR tube afforded clean oxidation to [LFeIII(MeCN)2]3+, with no apparent decomposition,81 after sitting at room temperature overnight, as indicated by 1H NMR spectroscopy (Figure S48). The deep blue solution was then refilled with ∼60 equiv. 15NH3 at −30 °C after being degassed by five cycles of freeze-pump-thaw. The solution was maintained at −30 °C for 2 hours, then warmed to room temperature overnight, followed by continuous mixing at room temperature for 36 hours. The reaction was complete, as indicated by the disappearance of the paramagnetic signals in the 1H NMR spectrum, along with a color change from intense blue to dark brown. The observation of 1H NMR spectroscopic signals of [LFeII(MeCN)2]2+ and [LFeII(NH3)(MeCN)]2+ indicate that the Fe complex survived under the reaction conditions (Figure S49). 15N2 was detected at −71.2 ppm vs Me15NO2 by 15N NMR spectroscopy (Figure S50), confirming NH3 as the source of the N2 product (Scheme 4).
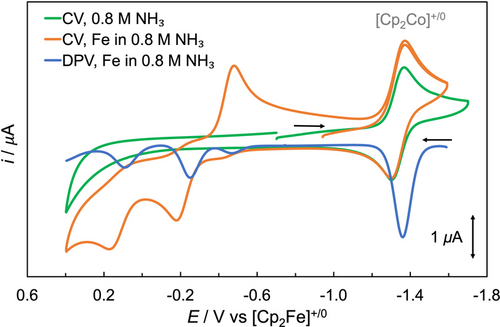
CV (orange) and DPV (blue) of [LFeII(MeCN)2]2+ in the presence of 0.8 M NH3 in MeCN with 0.1 M [n-Bu4N][B(C6F5)4] supporting electrolyte under 1 atm N2 at room temperature, and CV of 0.8 M NH3 (green) in MeCN for reference. [Cp2Co][PF6] was added as the internal standard. Scan rate=100 mV/s for the CV. Ep=E1/2–ΔEpulse/2 for DPV,74 with ΔEpulse representing the pulse amplitude (50 mV in our experiments).
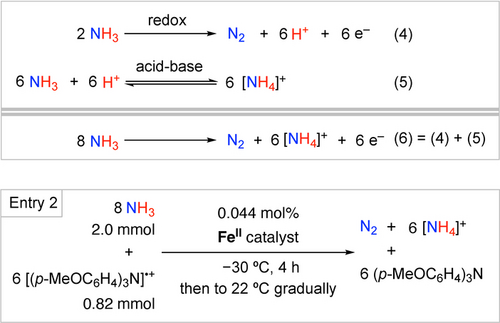
Stoichiometry of ammonia oxidation to N2, and an example of reaction conditions for chemical catalysis (Table 1, Entry 2).
After verifying the formation of N2 by 15N NMR spectroscopy in NMR tube experiments, we conducted larger-scale catalytic reactions to determine the yield of N2 by gas chromatography (GC) using a thermal conductivity detector (TCD) and helium as a standard for integration. Using the oxidant [(p-MeOC6H4)3N]⋅+ and NH3 in a ratio of 2.5, we achieved 91 turnovers of N2 per Fe (Table 1, Entry 1), indicating a 50 % increase in the yield of N2 compared to the results reported in Entry 5 of Table S5 (see detailed results of initial explorations with use of [(p-tolyl)3N]⋅+ (E1/2=0.38 V in MeCN)80 as the oxidant in the Supporting Information). The weak oxidant [(p-MeOC6H4)3N]⋅+ provides more flexibility and significant advantages in determining the optimal reaction temperature. Catalysis performed at −30 °C and −20 °C produced improved yields of N2, generating 104 and 98 turnovers per Fe, respectively (Table 1, Entries 2 and 3). However, further warming the reaction temperature afforded lower yields of N2, with 77 turnovers at −10 °C (Table 1, Entry 4), 59 turnovers at 0 °C (Table 1, Entry 5), and 25 turnovers at 22 °C (Table 1, Entry 6). These results underscore the value of low temperatures in this catalytic reaction, which may stabilize intermediates and thereby enhance the requisite N−N bond formation, as first reported in the Ru-catalyzed ammonia oxidation by Nishibayashi and co-workers,28 or improve the compatibility between oxidizing agents and free NH3. Moreover, the lower redox potential of [(p-MeOC6H4)3N]⋅+ compared to [(p-tolyl)3N]⋅+ reduces the driving force required for the catalysis, implying a lower “chemical overpotential”.
Entry |
FeII (mM) |
[(p-MeOC6H4)3N]⋅+ (M) |
NH3 (M) |
Tb (°C) |
Turnovers of N2 per Fe |
|---|---|---|---|---|---|
1 |
0.11 |
0.10 |
0.25 |
−40 |
91 |
2 |
0.11 |
0.10 |
0.25 |
−30 |
104 |
3 |
0.11 |
0.10 |
0.25 |
−20 |
98 |
4 |
0.11 |
0.10 |
0.25 |
−10 |
77 |
5 |
0.11 |
0.10 |
0.25 |
0 |
59 |
6 |
0.11 |
0.092 |
0.25 |
22 |
25 |
7 |
0.060 |
0.15 |
0.74 |
−30 |
148 |
8 |
0.043 |
0.10 |
1.05 |
−30 |
182 |
9 |
0 |
0.10 |
0.25 |
−30 |
0 |
- aAll the reactions were carried out by mixing [LFeII(MeCN)2][PF6]2 and [(p-MeOC6H4)3N⋅+][PF6]− in MeCN, followed by addition of an NH3 solution in MeCN at the temperature indicated. Reactions were vigorously stirred at low temperature for 4–6 hours, then gradually warmed to room temperature overnight. bThe temperature values represent a range of ±3 °C.
Increasing the concentration of the NH3/oxidant pair to a ratio of 5.0 further improved the turnovers of N2 per Fe to 148 (Table 1, Entry 7). By reducing the catalyst loading, a substantial increase in the yield of N2 was achieved, affording 182 turnovers of N2 per Fe (Table 1, Entry 8), which represents the highest reported thus far for the chemical catalysis of ammonia oxidation by a soluble molecular complex. Recharging the catalytic system with fresh (p-MeOC6H4)3N⋅+ and NH3 produced additional 2.4 turnovers of N2 per Fe (see details in the Supporting Information), implying nearly complete deactivation of the catalysts in the last round of catalysis. Note that in Table 1, Entry 8, an extremely low loading of catalyst (0.043 mM, 0.004 mol %) worked well, indicating that the Fe catalyst is highly efficient in chemical catalysis. Low loading of a Ru catalyst (0.5 mM, 0.0035 mol %) for electrocatalytic ammonia oxidation in aqueous solutions was recently reported by Ménard and co-workers.37 We emphasize that catalysis with low loadings of first-row transition metal catalysts44 is typically more challenging, as the d orbital energy splitting in first-row transition metal complexes is generally smaller than that in second- and third-row transition metals, and the difference is expected to contribute to the potentially greater difficulty in achieving high stability of first-row transition metal catalysts in the presence of ammonia. Control experiments in the absence of Fe catalyst show no N2 formation (Table 1, Entry 9).
Electrocatalytic Ammonia Oxidation
We examined [LFeII(MeCN)2]2+ as a potential electrocatalyst to compare to the chemical catalysis of ammonia oxidation described above. Electrochemical properties of [LFeII(MeCN)2]2+ in the presence of NH3 were evaluated using a solution of 1.0 mM FeII and 0.8 M NH3 in MeCN. In the presence of NH3, three redox events were observed on the anodic scans of CV and DPV (Figure 4), corresponding to redox potentials of [LFeII(NH3)2]2+, [LFeII(NH3)(MeCN)]2+, and [LFeII(MeCN)2]2+, respectively, in agreement with the electrochemical results obtained with the isolated [LFeIII(NH3)2]3+ above. On the return cathodic scan, the predominant reduction wave corresponds to [LFeIII(NH3)2]3+. The irreversibility observed in all three redox waves implies an electron transfer-chemical (EC) process involving the oxidation-induced replacement of axial MeCN ligands by NH3, driven by the more favorable binding of NH3 vs. MeCN to the FeIII relative to the FeII center, as demonstrated above. No clear evidence was obtained for PCET events during the anodic scan (see details in the Supporting Information).
Based on the apparent lack of electrocatalysis when replacing the chemical oxidant with an electrode, we next explored the feasibility of mediated electrocatalytic ammonia oxidation using [(p-MeOC6H4)3N], which can be electrochemically oxidized to generate its radical cation. CVs in MeCN at 295 K and 1 atm N2 in the absence and presence of NH3 are shown in Figure 5. In the absence of NH3, FeII complex (light orange) and (p-MeOC6H4)3N (light blue) showed quasi-reversible redox events at nearly identical potentials (0.15 V). A solution of 1.0 mM FeII complex and 2.0 equiv. (p-MeOC6H4)3N reveals an increase in peak current (light red), which was merely the sum of the peak current observed independently from 1.0 mM FeII complex and from 2.0 mM (p-MeOC6H4)3N (Table S8).
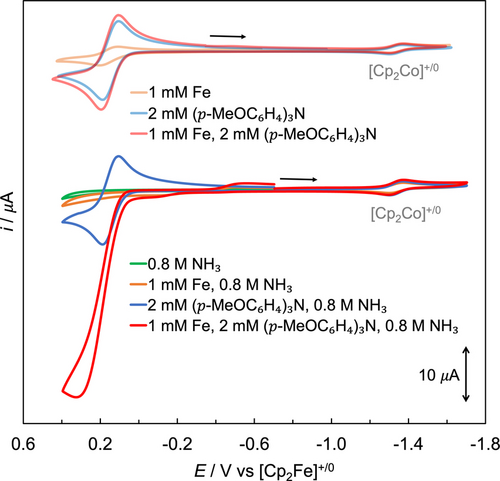
CVs in MeCN (0.1 M [n-Bu4N][B(C6F5)4] supporting electrolyte) at 295 K and 1 atm N2 of 1.0 mM [LFeII(MeCN)2]2+ (top CVs, light orange), 2.0 mM (p-MeOC6H4)3N (top CVs, light blue), 1.0 mM [LFeII(MeCN)2]2+ and 2.0 mM (p-MeOC6H4)3N (top CVs, light red), 0.8 M NH3 (bottom CVs, green), 1.0 mM [LFeII(MeCN)2]2+ and 0.8 M NH3 (bottom CVs, orange), 2.0 mM (p-MeOC6H4)3N and 0.8 M NH3 (bottom CVs, blue), as well as the solution containing 1.0 mM [LFeII(MeCN)2]2+, 2.0 mM (p-MeOC6H4)3N and 0.8 M NH3 (bottom CVs, red). [Cp2Co][PF6] was added as the internal standard. Scan rate=100 mV/s.
In the presence of NH3, FeII complex displayed three redox events (orange, see above for details), while (p-MeOC6H4)3N still exhibited a quasi-reversible redox event at 0.15 V (blue). A solution containing 1.0 mM FeII complex, 2.0 equiv. (p-MeOC6H4)3N and 0.8 M NH3 in MeCN revealed a significant current enhancement, with a ratio of 3.7 for icat/i at room temperature (red trace, icat represents the maximum current of catalytic waves), implying electrocatalytic oxidation of NH3 synergistically enhanced by both the Fe catalyst and (p-MeOC6H4)3N (see CVs in Figure S64 for electrocatalysis at −20 °C). Ecat/2, defined as the potential at which the catalytic wave reaches half of its maximum current (icat/2),82 was observed at 0.18 V in MeCN (red trace in Figure 5). In this CV experiment, [LFeII(MeCN)2]2+, [LFeII(NH3)(MeCN)]2+ and [LFeII(NH3)2]2+ were present in the bulk solution. While E1/2 of [LFeII(MeCN)2]2+ (0.15 V) is in close proximity to Ecat/2, it is unlikely that the FeIII/II redox potential directly accounts for the catalytic current response, as neither [LFeII(MeCN)2]2+ nor [LFeIII(MeCN)2]3+ have coordinating NH3 ligands. Instead, the E1/2 values of [LFeII(MeCN)2]2+ (0.15 V), [LFeII(NH3)(MeCN)]2+ (−0.15 V), and [LFeII(NH3)2]2+ (−0.47 V) collectively govern the formation of their respective FeIII species, all of which are likely rapidly transformed to [LFeIII(NH3)2]3+ in the presence of NH3. We speculate that the NH3-bound Fe complexes cooperate with (p-MeOC6H4)3N⋅+, which has an E1/2 of 0.15 V that also closely aligns with Ecat/2, thereby electrochemically catalyzing the oxidation of NH3 (see details in the proposed mechanism below).
The overpotential was estimated using the equation η=Ecat/2–E°N2/NH3.82, 83 Our observed Ecat/2 value was +0.18 V. E°N2/NH3 is the thermodynamic equilibrium potential of the half reaction shown in eq. 6 (Scheme 4) under standard-state conditions at 298 K in MeCN, with a value of −0.94 V.84 The resulting value of the overpotential is approximately 1.1 V. It should be noted that the conditions are not identical for Ecat/2 (0.8 M NH3) compared to E°N2/NH3 (1 : 1 buffered condition of 1 M NH3 and 1 M [NH4+]), so the overpotential should be viewed as an estimate, not an absolutely accurate value. However, the impact of this discrepancy is expected to be small, relative to the magnitude of the overpotential.82
The onset potential (Eonset),85 is defined as the point at which the baseline current intersects with the linear extrapolation of the steepest portion of the rising current of the catalytic wave. Eonset of the catalytic wave was observed at 0.09 V in MeCN (Figure S59), which is ∼250 mV (estimated error: ±30 mV) more cathodic than the onset potential of uncatalyzed background ammonia oxidation on a glassy carbon electrode (Figure S61, Eonset=0.34 V). The catalytic Eonset occurs at a milder potential than that observed for [(TPA)FeII(MeCN)2]2+ (0.70 V in MeCN),29 [(bpyPy2Me)FeII(MeCN)2]2+ (0.45 V in MeCN),30 and [(BPMMeO)Fe(MeCN)2]2+ (0.29 V in MeCN),38 yet it occurs at a higher potential than that reported for [Cp*FeIII(1,2-Ph2PC6H4NH)(NH3)]+ (−0.04 V in THF)34 or [iPr2NNF6]CuI(NH3) (−0.24 V in MeCN).35
Controlled-potential electrolysis (CPE) was performed to quantify the product of the mediated electrocatalysis. Initially, a CPE experiment was carried out at room temperature with a constant potential of 0.25 V vs [Cp2Fe]+/0, using a solution containing 0.12 mM FeII catalyst, 0.24 mM (p-MeOC6H4)3N and 0.8 M NH3. After 3 hours, 5.7 Coulombs of charge had passed (Figure S66). GC analysis revealed 1.8 turnovers of N2 per Fe were produced in the working electrode compartment, with a Faradaic efficiency of 16 % for N2. A CV recorded after the electrolysis showed that the redox wave of (p-MeOC6H4)3N disappeared (Figure S65), indicating its decomposition during CPE at room temperature, suggesting an explanation for the low Faradaic efficiency.
Subsequently, the CPE was performed at low temperatures, as reported above for the chemical catalysis. Using the same reaction solution as for the CPE at room temperature, a potential of 0.20 V vs [Cp2Fe]+/0 was applied at −20±3 °C for 4 hours, at which point 6.4 Coulombs of charge had passed (Figure S68). A CV recorded after electrolysis demonstrated that the redox potential of the internal standard, [Cp2Co][PF6], drifted catholically by about 120 mV, indicating that the applied potential shifted from 0.20 V initially to a final potential of 0.32 V at the end of the CPE. Additionally, the previously observed current enhancement on the cyclic voltammogram prior to CPE was no longer present (Figure S67). However, a quasi-reversible oxidation peak of (p-MeOC6H4)3N remained, indicating that (p-MeOC6H4)3N and its radical cation at least partially survived at −20 °C during catalysis, consistent with the observation of characteristic bright blue color of the radical cation during bulk electrolysis.
The headspace of the working electrode compartment was sampled while the solution was maintained at −20 °C. GC analysis revealed that 7.2 turnovers of N2 per Fe were produced, confirming that the catalytic oxidation of NH3 to form N2 occurred at low temperatures. The Faradaic efficiency for N2 generation was determined to be 61 %. The reaction mixtures were then warmed to room temperature; headspace analysis of the working electrode compartment showed that 9.3 turnovers of N2 per Fe were produced, giving a Faradaic efficiency of 75 % for N2. The increased N2 was released from the reaction mixture after warming from −20 °C to room temperature. Additionally, the headspace of the counter electrode compartment was also sampled when the reaction mixtures were left at room temperature. GC analysis revealed 40.5 turnovers of H2 per Fe were generated, corresponding to a Faradaic efficiency of 100 % for H2. The ratio of H2/N2 is approximately 4.4. Control experiments conducted in the absence of either (p-MeOC6H4)3N or FeII catalyst showed no evidence for N2 formation (Table S10).
In contrast to the chemical catalysis of ammonia oxidation in the reactions of [LFeII(NH3)2]2+, (p-MeOC6H4)3N⋅+ and NH3 solution in MeCN at low temperatures, e.g., Entry 3 in Table 1, the electrocatalysis synergistically promoted by [LFeII(NH3)2]2+ and triarylamines produced substantially reduced turnovers of N2 per Fe. We attribute this observation to two factors. First, the chemical catalytic reactions occurred over 12–16 hours, while the bulk electrolysis experiments were carried out for 4 hours, as electrocatalysis seems more sensitive to temperature perturbations compared to chemical catalysis. Since the catalysis of ammonia oxidation is slow, an extended reaction time could produce an increased yield of N2. Second, it is plausible that electrode passivation occurred as the Fe catalyst and/or triarylamine degraded during bulk electrolysis, consequently leading to a lower turnover of N2 in electrocatalysis. This possible passivation is also consistent with difficulty we encountered in obtaining CVs after the bulk electrolysis when the voltammetry electrode remained in the solution during electrolysis.
Mechanistic Studies
We investigated the deprotonation of [LFeIII(NH3)2]3+ using 1H NMR spectroscopy. When 10 equiv. of NH3 were added to [LFeIII(NH3)2]3+ at room temperature, some of the paramagnetic [LFeIII(NH3)2]3+ was transformed overnight into diamagnetic [LFeII(MeCN)2]2+ (Figure S38, see experiments with sterically hindered stronger bases in Scheme S9 in the Supporting Information). The deprotonated species [LFeIII(NH3)(NH2)]2+ was not observed. This observation suggests that the first deprotonation step is either slow or thermodynamically unfavored, and is followed by a thermodynamically favored, fast conversion of the transient [LFeIII(NH3)(NH2)]2+ to [LFeII(MeCN)2]2+ (Scheme S9). Thermal instability of an FeIII−NH2 species was observed previously.20 The slow deprotonation of [LFeIII(NH3)2]3+ may account for the absence of current enhancement on the CV timescale for a solution of [LFeII(MeCN)2]2+ and NH3 in MeCN.
In the triarylamine-mediated electrocatalysis using [LFeIII(NH3)2]3+, 2.0 equiv. of triarylamine, and excess NH3 in MeCN, triarylamine is not expected to be capable of accelerating the direct deprotonation, as it is significantly less basic than NH3 (for reference, pKa=1.28 for [Ph3NH+]78 vs pKa=16.46 for [NH4+]79 in MeCN). The oxidized triarylamine can function as an electron shuttle (Scheme 5), accelerating heterogeneous electron transfers between the electrode and the soluble molecular Fe catalyst (ΔEp=81 mV at 0.1 V/s for (p-MeOC6H4)3N in Figure S56). In addition, it is possible that the electrochemically generated (p-MeOC6H4)3N⋅+ could facilitate a homogeneous PCET process in conjunction with NH3.
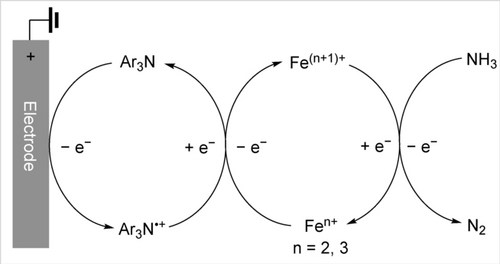
Electrochemical oxidation of Fe complexes accelerated by Ar3N⋅+ as a molecular mediator; Ar3N=(p-MeOC6H4)3N.
DFT computations were used to complement experimental mechanistic studies and to assess potential pathways. Calculated reduction potentials of [(L')FeII(NH3)]2+ (L'=MeCN or NH3) are in relatively good agreement with experimental values at the level of theory used (see Methods in the Supporting Information).
For [(L')FeII(NH3)]2+, both the solvent-bound species and the bis-ammine may be present, as they are close in energy (ΔG=−1.4 kcal/mol, Scheme S14). When L'trans=MeCN, oxidation of [FeII(MeCN)(NH3)]2+ followed by deprotonation from the oxidized species [FeIII(MeCN)(NH3)]3+ is most favorable, as it has a calculated pKa of 25.1 (Scheme 6a, orange values). Deprotonation followed by oxidation is unfavored, as this pathway has a much higher pKa than that of NH3. The N−H BDFE of the FeII−NH3 complex is calculated to be 82.1 kcal/mol. It is important to note that while [Ar3NH]+ alone has a calculated BDFE of 57.0 kcal/mol, the Ar3N/[NH4]+ couple has an effective formal BDFE of 78.9 kcal/mol for a net hydrogen atom transfer (HAT) in MeCN.86 The HAT from [FeII(MeCN)(NH3)]2+ to the oxidant-base pair of Ar3N⋅+ and NH3 is uphill by only 3.2 kcal/mol. Similar trends are seen when L'trans=NH3 (Scheme 6a, blue values). The oxidation to form [FeIII(NH3)2]3+ is very favorable at −0.41 V vs [Cp2Fe]+/0. Subsequent deprotonation of the oxidized species [FeIII(NH3)2]3+ is feasible, as the calculated pKa (27.4) is very close to the range identified experimentally (Scheme S9) and agrees with the experimental observation of initial deprotonation as the slowest step. Deprotonation of [FeII(NH3)2]2+ is not feasible, as demonstrated by its high pKa of 45.7. The calculated BDFE of 76.5 kcal/mol is lower in energy when L'trans=NH3 and is less than the BDFE of the Ar3N/[NH4]+ couple, allowing for HAT from [FeII(NH3)2]2+ to afford [FeIII(NH2)(NH3)]2+.
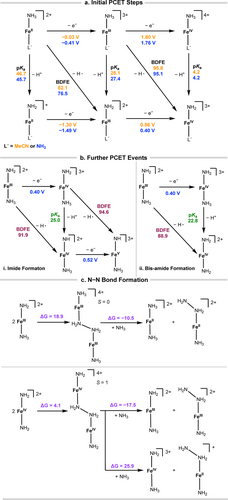
a) Initial PCET steps from tetra-NHC Fe complexes with MeCN or NH3 as the trans ligand. Values are in orange for L'trans=MeCN, and blue for L'trans=NH3. BDFEs are reported in kcal/mol, and reduction potentials are reported as V vs [Cp2Fe]+/0. b) Further PCET events can occur (i) from the amidyl moiety, wherein the trans NH3 acts as a spectator, or (ii) from the trans NH3 to form the bis(amide). c) Bimetallic coupling pathways, with ΔG values in kcal/mol. The cyclic tetra-NHC ligand (L) is not shown. All energies were computed with MeCN solvation.
It is likely that most of the catalysis operates from [FeIII(NH2)(NH3)]2+ where L'trans=NH3, since NH3 exchange with MeCN is facile, especially at higher oxidation states. The potential for oxidation is calculated to be 0.40 V vs [Cp2Fe]+/0 for the formation of [FeIV(NH2)(NH3)]3+, which is otherwise energetically unfavorable through HAT from [FeIII(NH3)2]3+ (BDFE of N−H bond=95.1 kcal/mol).
Further PCET events from [FeIII(NH2)(NH3)]3+ can occur from the amidyl moiety or from the trans NH3 ligand (Scheme 6b). A pathway through an amide species has been previously invoked,13, 23, 87, 88 and the formation of a bis-amide has been observed in second and third row transition metals.24 For the formation of the FeIV imide complex in Scheme 6b-i, deprotonation of [FeIV(NH2)(NH3)]3+ to form [FeIV(NH)(NH3)]2+ has a calculated pKa of 25.0. The N−H BDFE for FeIII−NH2 is calculated to be 91.9 kcal/mol, which is higher than previously calculated steps. The BDFE to generate [FeV(NH)(NH3)]3+ directly from [FeIV(NH2)(NH3)]3+ was calculated to be 94.6 kcal/mol, which is even more unfavorable. Alternatively, deprotonation could occur from the trans NH3 ligand to form an FeIV bis-amide species, [FeIV(NH2)2]2+ (Scheme 6b-ii). This has a lower calculated pKa and is lower in energy by 3.0 kcal/mol (1.37ΔpKa), compared to deprotonation of the amidyl group. The overall N−H BDFE for trans NH3 is still somewhat high at 88.9 kcal/mol, though it may be lowered by favorable ion pairing. Detailed evaluation of explicit solvent and counterions will be examined in future studies.
Despite the high oxidation state, FeIV complexes have been recently invoked in catalytic ammonia oxidation. In calculations by Peters and co-workers for electrocatalytic ammonia oxidation using a dicationic catalyst [(bpyPy2Me)FeII(MeCN)2]2+, high-valent FeIV and FeV intermediates were proposed to account for N−N bond formation.30 In recent work by Wang, Liao, Ye, and co-workers, evidence was presented suggesting that their electrocatalytic ammonia oxidations involved intermediates featuring FeIV and FeV species.34
For N−N bond formation, it is possible that [FeIII(NH2)(NH3)]2+ could couple to form a bridged bimetallic hydrazido complex; a similar mechanism was invoked in catalysis by (TMP)Ru catalysts (TMP=tetramesitylporphyrin).40 This N−N bond formation is found to be unfavorable by 18.9 kcal/mol (Scheme 6c, upper). With each species possessing 2+ charge, however, it may be electrostatically unfavorable, even in a polar solvent like MeCN. Additionally, the formation of the partial π-bond might overstabilize the amide and render it unreactive (see below).89 Alternatively, bimetallic coupling could occur from [FeIV(NH2)2]2+ (Scheme 6c, lower). The formation of the bridged complex has a much lower energy (4.1 kcal/mol). From the bridged states, amido transfer can occur by either breaking the Fe−NH2 bond homolytically or heterolytically. In the bridged FeIIINH2NH2FeIII complex, the homolytic route is favorable by 10.5 kcal/mol, whereas in the FeIVNH2NH2FeIV complex, it is favorable by 17.5 kcal/mol. The heterolytic reaction from the bridged FeIVNH2NH2FeIV species is unfavorable by almost 26 kcal/mol, rendering that pathway prohibitively high in energy. Following N−N formation, “NH2−NH2” could be easily oxidized to N2 under the catalytic conditions, since the N−H bond of free hydrazine has a much lower first BDFE (72.6 kcal/mol in the gas phase vs 99.4 kcal/mol for NH3)86 and the electrochemical oxidation of hydrazine is 433 mV more favorable than oxidation of ammonia, based on E° in MeCN with NH3 as the base.84 Warren and co-workers observed facile oxidation of free NH2−NH2 to N2 in MeCN solution with an onset potential of −0.40 V on a glassy carbon electrode.35 In the case of [FeIII(NH2NH2)(NH2)]2+, HAT may even be able to proceed via direct Ar3N+⋅ mediation, considering its weak hydrazido N−H bonds.86, 90 The [FeIII(NH2)(NH3)]2+ would then enter back into the catalytic cycle.
The macrocyclic tetra-NHC ligand plays a key role as a strong donor, and may also help stabilize the [LFeIII(NH2NH2)(NH2)]2+ complex. In previous computations with (TPP)Fe(NH3)(NH2) (TPP=tetraphenylporphyrin) complexes of the same oxidation state and multiplicity, the amidyl moiety is nonplanar, with considerable electron density causing the hydrogen atoms to move out of the plane.40, 87 As shown in Figure 6, the amidyl moiety is planar; similar geometries were also seen for computed structures of [LFeIII(NH3)(NH2)]2+ and [LFeIV(NH2)2]2+ (Figure S77 and S78). By natural bond orbital (NBO)91 analysis, the Fe−NH2 bond in both molecules consists of one fully occupied σ orbital and one partially occupied π orbital, suggesting the initial formation of the metal-ligand π-system. This bonding is thought to stabilize the metal-amidyl complexes89 and is likely the source of the planar geometry. These results also illustrate the impact of macrocyclic ligand choice compared to previously studied Fe complexes.87, 88
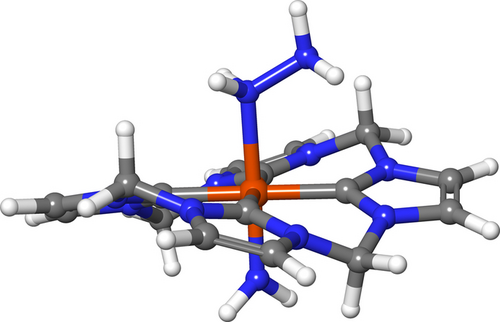
Computed structure of [LFeIII(NH2NH2)(NH2)]2+ with its hydrazido ligand oriented towards the macrocycle.
The terminal nitrogen atom of the hydrazido ligand is oriented towards the C−H bond of the macrocyclic ligand. In the NBO framework, hydrogen bonding can be treated as a charge transfer between the lone pair of the nitrogen (hydrogen bond acceptor) and the σ* orbital of the C−H bond (hydrogen bond donor).92 Our computations reveal a weak hydrogen bonding interaction that stabilizes the complex by 1 kcal/mol. There is a small amount of charge transfer present, though about an order of magnitude weaker than previously been observed between anions and O−H bond of water.93 The lowest energy electronic state of [LFeIII(NH2NH2)(NH2)]2+ is a doublet wherein most spin density is located on the Fe center, while some is located on the nitrogen of the trans-NH2 ligand.
Finally, the possibility of NH3 attack on [LFeIII(NH2)(NH3)]2+ was explored as an alternative pathway. In this reaction, a proton must be lost from the attacking NH3. This deprotonation can be done by the tetra-NHC ligand or an additional ammonia, with energetic costs of 52.2 and 65.7 kcal/mol, respectively. Neither of these paths are feasible. It should be noted that there is an energetic cost of 10.7 kcal/mol for the approach of the additional ammonia to the [LFeIII(NH2)(NH3)]2+ species, which contributes to this large energy. NH3 attack at later states was not considered in this work, though it will be the subject of future studies.
Conclusion
We have shown that an Earth-abundant Fe complex catalyzes the oxidation of ammonia to N2, even at very low catalyst loading and at low temperatures, achieving the highest reported turnover number of N2 in chemical catalysis by soluble molecular catalysts. Chelated in a cyclic tetra-NHC ligation environment, the Fe catalyst exhibited an enhanced stability against demetallation that can be caused by binding of NH3 to the metal, leading to loss of the supporting ligand. Taking advantage of the one-electron redox reversibility of triarylamines, we translated the chemical catalysis to an unprecedented electrocatalytic oxidation of ammonia that is cooperatively promoted by a transition metal catalyst and an organic mediator. Though further improvement is still needed for this mediated electrocatalysis in terms of overpotentials and catalytic turnovers, this new approach represents a promising avenue for advancing the electrocatalytic oxidation of ammonia and broadens the horizons of reaction types to consider, given the accessibility of a versatile range of molecular mediators.
Supporting Information
The authors have cited additional references within the Supporting Information.94-138
Acknowledgments
This research was supported as part of the Center for Molecular Electrocatalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences (BES). We thank Dr. Evan A. Patrick for assistance with collection and analysis of the XRD structural data, and gratefully acknowledge Dr. Alexander S. Phearman for insightful discussions and helpful technical expertise.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.