Total Synthesis of (−)-Batrachotoxin Enabled by a Pd/Ag-Promoted Suzuki–Miyaura Coupling Reaction
Abstract
Batrachotoxin is an extremely potent cardio- and neurotoxic steroidal alkaloid found in certain species of frogs, birds, and beetles. The steroidal 6/6/6/5-membered carbocycle (ABCD-ring) is U-shaped and functionalized with two double bonds, a six-membered C3-hemiacetal across the AB-ring, a seven-membered oxazepane on the CD-ring, and a dimethylpyrrolecarboxy group at the D-ring carbon chain. These structural features present an unusual and formidable synthetic challenge. Herein we report a total synthesis of batrachotoxin based on a newly devised convergent strategy through a 22-step sequence. Enantiopure AB-ring and D-ring fragments were prepared and subjected to a crucial C(sp2)−C(sp2) coupling reaction. Although both C(sp2) centers were sterically encumbered by proximal tetrasubstituted carbon atoms, Ag2O strongly promoted the Pd(PPh3)4-catalyzed Suzuki–Miyaura coupling reaction at room temperature, thereby connecting the two fragments without damaging their preexisting functionalities. Subsequent treatment with t-BuOK induced Dieckmann condensation to cyclize the C-ring. The judiciously optimized functionalizations realized oxazepane formation, carbon chain extension, and pyrrole carboxylic acid condensation to deliver batrachotoxin.
Introduction
For centuries, skin secretions from brightly colored frogs of the genus Phyllobates in the Choco rain forest of Colombia have been used to prepare deadly darts for blowguns.1 Batrachotoxin (1, Scheme 1) and batrachotoxinin A (2) were isolated as the toxic principles of Phyllobates and structurally determined to be uniquely functionalized steroidal alkaloids by Daly and Witkop in 1968.2 Whereas the name was derived from the Greek “batrachos,” meaning frog, these alkaloids were later also identified in toxic New Guinea birds and beetles.3 Compound 1 is among the most toxic natural substances known (LD50, subcutaneous in mice, 2 μg/kg), and 2 retains only 1/500 of the toxicity of 1 (LD50, 1 mg/kg), indicating the biological significance of the 2,4-dimethyl-1H-pyrrole-3-carboxy group at the C20 position. The potent toxicity of 1 originates from its strong agonistic activity of voltage-gated sodium channels,4 which orchestrate the generation of action potentials and are key elements in the signal transduction process of nerve and muscle membranes. Because of its specific function, 1 is widely utilized to study the functions of sodium channels.5, 6
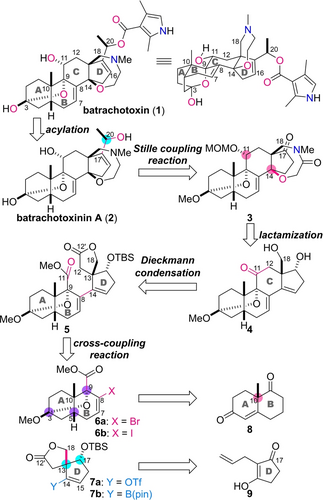
Structure of batrachotoxin (1) and retrosynthesis of 1. MOM=methoxymethyl, Tf=trifluoromethanesulfonyl, pin=pinacolate.
The complex architecture of 1 has multiple intriguing features that are not shared by common steroidal natural products.7 The 6/6/6/5-membered ABCD-ring carbocycle has a characteristic U-shape due to the atypical cis-fusion of the AB- and CD-rings. The carbocycle is adorned with C7=C8 and C16=C17 double bonds, a C9α-oxygen functionality that forms the six-membered C3-hemiacetal across the AB-ring, C18-nitrogen and C14-oxygen functionalities that constitute a seven-membered oxazepane on the CD-ring, and a C17-carbon chain that appends the dimethylpyrrolecarboxy group. This unusual array of diverse functional groups poses a formidable synthetic challenge.8 Witkop and co-workers derivatized 2 to 1 by esterifying dimethylpyrrolecarboxylic acid in 1969,2b and Wehrli and co-workers reported a semisynthesis of 2 from progesterone in 45 steps (0.0022 % yield) in 1972.9 Following these early synthetic studies, various synthetic routes to 1 and 2 continued to be pursued for more than 50 years.10, 11 In 1998, the Kishi group achieved the first total synthesis of 2 from Wieland-Miescher ketone in 48 steps (0.70 % yield), thus also completing the formal synthesis of 1.12 More recently, the Du Bois group constructed 1 in 2016 (24 steps, 0.24 %)13 and the Luo group assembled 2 in 2020 (16 steps, 0.19 %),14 both starting from Hajos-Parrish ketone. These three total syntheses not only highlight the creativity of the investigators but have also enriched the science of organic synthesis.
As part of our continued interest in realizing total syntheses of bioactive and densely oxygenated steroids,15 we became interested in devising an efficient strategy for expeditiously constructing the unique structure of 1. Herein we describe a novel convergent strategy using the Suzuki–Miyaura coupling and Dieckmann condensation reactions, by which we realized the total synthesis of batrachotoxin (1) in 22 steps as the longest linear sequence (0.71 % yield). Because of its simplicity and efficacy, the newly designed route to 1 should provide new valuable information for the syntheses of numerous structurally complex steroids.
Results and Discussion
A convergent strategy was planned for the total synthesis of 1, as the direct coupling of functionalized substructures is generally more advantageous toward realizing a shorter synthetic route (Scheme 1). Therefore, we retrosynthetically disassembled 1 into AB-ring 6 and D-ring 7 by disconnecting the C11−C12 and C8−C14 bonds of the C-ring. These fragments together carry five of the eight stereocenters of 1, including the C10 and C13 quaternary centers. Accordingly, the use of these functionalized chiral fragments was expected to simplify the stereochemical manipulation after convergent assembly. Compounds 6 and 7 were envisioned to be prepared from commercially available (+)-Wieland-Miescher ketone (8) and 2-allyl-3-hydroxycyclopent-2-en-1-one (9), respectively, through installing the C3, C5, C9, C13, and C17 stereocenters (indicated by purple and cyan circles). In the synthetic direction, the bond between C8 of 6 and C14 of 7 would be forged by a transition metal-catalyzed C(sp2)−C(sp2) coupling reaction. Because the C8 and C14 positions are sterically shielded by the neighboring C9- and C13-tetrasubstituted carbons, respectively, the intermolecular formation of the C8−C14 bond is a daunting task. This consideration led us to design the spirally fused five-membered lactone of 7 to minimize the negative steric effect of the two carbon chains at the C13 position. Furthermore, bromide 6 a, iodide 6 b, triflate 7 a, and pinacol boronate 7 b were to be prepared for screening the reaction conditions to attain the high-yielding generation of 5 from the AB-ring and D-ring fragments. After the coupling reaction, the C12′- and C11-carbonyl groups would be readily utilized for C-ring cyclization by the Dieckmann condensation. The subsequent decarboxylation reaction would expel the extra C12′-carbon and liberate the C18-hydroxy group, leading to 4. The C11 and C14 stereocenters were then to be constructed through C11 reduction and C14 hydroxylation, and the subsequent formation of the oxazepane ring would afford 3. Finally, carbon chain extension at the C17 position by a Stille coupling reaction and introduction of the last remaining C20 stereocenter would provide batrachotoxinin A (2), whose acylation would deliver the targeted batrachotoxin (1).
To prepare AB-ring fragments 6 a and 6 b (Scheme 2), the C5-olefin of (+)-Wieland-Miescher ketone (8, 99 % ee)16 was first hydrogenated by catalysis of Pd/C to stereoselectively introduce the C5-hydrogen of cis-fused decalin 10. By exploiting the different steric environments of the C3- and C9-carbonyl groups, the less hindered C3-ketone of 10 was protected as the 1,3-dioxolane ring of 11. The unreacted C9-ketone of 11 was then used to construct the C8-olefin of 12. Namely, sulfoxide was attached at the C8 position by the reagent combination of KH and PhS(O)OMe,17 and subsequently eliminated by treatment with Na2CO3 in heated toluene, furnishing enone 12. Dibromination of the olefin by Br2 in CCl4, followed by pyridine-promoted E1cB elimination converted 12 into α-bromo enone 13. Next, (1-ethoxyvinyl)lithium stereoselectively attacked the C9-ketone of 13 from the convex face, giving rise to adduct 14 as a single diastereomer. The resultant C9-tertiary alcohol participated in the internal acetalization in the presence of (+)-CSA and CH(OMe)3 in MeOH, resulting in the formation of methyl acetal 15 with the concomitant change of OEt with OMe at the C11 position. RuCl3-catalyzed oxidation of diene 15 with NaIO418 chemoselectively cleaved the more electron-rich C11-olefin to produce the brominated AB-ring 6 a. Alternatively, the iodinated AB-ring 6 b was derivatized from 15 over two steps; halogen-lithium exchange and in situ iodination, and ensuing RuCl3/NaIO4-oxidative olefin cleavage.
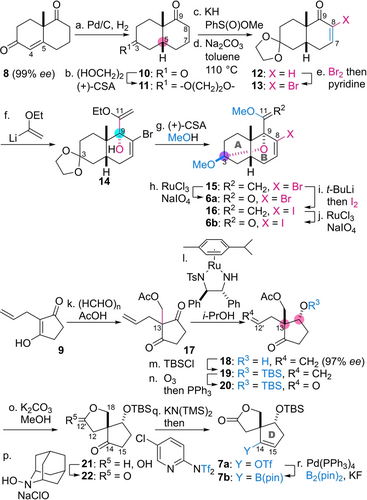
Preparation of AB-ring 6 a/6 b and D-ring 7 a/7 b. Reagents and conditions: a) Pd/C, H2, EtOAc, 25 °C; b) (HOCH2)2, (+)-10-camphorsulfonic acid ((+)-CSA; 10 mol%), benzene, 25 °C, 72 % (two steps from 8); c) KH, PhS(O)OMe, THF, 0 °C to 22 °C; d) Na2CO3, toluene, 110 °C, 94 % (two steps from 11); e) Br2, CCl4, 0 °C, then pyridine, 0 °C to 21 °C, 97 %; f) ethyl vinyl ether, t-BuLi, THF, −78 °C; g) (+)-CSA (10 mol%), CH(OMe)3, MeOH, 23 °C, 58 % (two steps from 13); h) RuCl3⋅nH2O (10 mol%), NaIO4, MeCN, pH 7 phosphate buffer, 0 °C to 22 °C, 49 % (three steps from 13, [6 a (C11−OMe):6 a′ (C11−OEt)=8 : 1]; i) t-BuLi, THF, −78 °C, then I2, −78 °C; j) RuCl3⋅nH2O (10 mol%), NaIO4, MeCN, pH 7 phosphate buffer, 0 °C to 23 °C, 72 % (two steps from 15); k) (HCHO)n, p-toluenesulfonic acid monohydrate (TsOH⋅H2O) (30 mol%), AcOH, 65 °C, 24 %; l) [(R,R)-N-(2-amino-1,2-diphenylethyl)-p-toluenesulfonamide](p-cymene)ruthenium(II) (10 mol%), i-PrOH, 25 °C; m) tert-butyldimethylsilyl chloride (TBSCl), imidazole, CH2Cl2, 24 °C, 63 % (two steps from 17); n) O3, CH2Cl2, −78 °C, then PPh3, −78 °C to 26 °C; o) K2CO3, MeOH, 0 °C, 88 % (two steps from 19); p) 2-hydroxy-2-azaadamantane (AZADOL) (10 mol%), n-Bu4NBr (10 mol%), KBr (10 mol%), aq. NaClO, CH2Cl2, aq. NaHCO3, 0 °C, 91 %; q) potassium bis(trimethylsilyl)amide (KN(TMS)2) (1 equiv), 2-[N,N-bis(trifluoromethylsulfonyl)amino]-5-chloropyridine (1 equiv), THF, −78 °C, 70 %; r) Pd(PPh3)4 (5 mol%), bis(pinacolato)diboron (B2(pin)2), KF, THF, H2O, 25 °C, 89 %.
Preparation of D-ring fragments 7 a and 7 b commenced with the acetoxy methylation of ketone 919 using paraformaldehyde in AcOH (Scheme 2).20 The reaction installed the C13 quaternary center of diketone 17. The next Ru-catalyzed asymmetric transfer hydrogenation in i-PrOH21 effectively desymmetrized meso compound 17 by reducing one of the two ketones, thereby affording 18 (97 % ee) along with its minor C13-diastereomer (dr=4 : 1). The inseparable diastereomixture was subjected to TBSCl and imidazole in CH2Cl2 to provide single diastereomer 19 after silica gel column purification. Sequential treatment with O3 and PPh3 transformed olefin 19 to aldehyde 20, and basic methanolysis of 20 induced the acetyl removal and lactol formation, thereby internally protecting the C18-hydroxy group of 21. AZADOL22 was then used as a catalyst for the oxidation of lactol 21 to spiral lactone 22. The triflated D-ring 7 a was chemoselectively produced from 22 by taking advantage of the lower pKa of the five-membered ketone than of the lactone. Specifically, one equivalent of KN(TMS)2 allowed for site-selective deprotonation at C15 over C12 to generate the C14-enolate, which was trapped with 2-[N,N-bis(trifluoromethylsulfonyl)amino]-5-chloropyridine to furnish 7 a.23 The boronated D-ring 7 b was in turn derived from 7 a by employing a catalytic amount of Pd(PPh3)4 and a stoichiometric amount of B2(pin)2. Hence, the syntheses of enantiopure fragments 6 a, 6 b, 7 a, and 7 b were completed in 8, 9, 7, and 8 steps, respectively.
The stage was now set for screening the conditions to couple oxa-bridged AB-ring 6 a/6 b and spirally fused D-ring 7 a/7 b with the various potentially reactive functionalities (Table 1).24 We previously applied a powerful Pd/Ni-mediated cross-electrophile reaction developed by Weix25 to a related system and cyclized the steroidal C-ring in high yield.11 Despite the success of the intramolecular reaction, the intermolecular counterpart between bromide 6 a and triflate 7 a was significantly less effective regardless of the use of stoichiometric amounts of Pd and Ni reagents (entry 1). When 6 a (1 equiv) and 7 a (1 equiv) were subjected to NiBr2⋅diglyme (2 equiv), 2,2′-bipyridine (2 equiv), PdCl2 (2 equiv), dppp (2 equiv), and Zn (5 equiv) in DMF at 80 °C (entry 1), the desired adduct 5 was obtained in only 19 % yield. Changing the AB-ring fragment from bromide 6 a to iodide 6 b negligibly affected the results, generating 5 in 18 % yield (entry 2). While 6 a/6 b and 7 a were fully consumed in these reactions, protodehalogenated AB-ring 6 c, protodetriflated D-ring 7 c, and D-ring dimer 23 were isolated. The results corroborated the intrinsic difficulty of the coupling even when applying the sterically minimized spiral ring system 7 a.
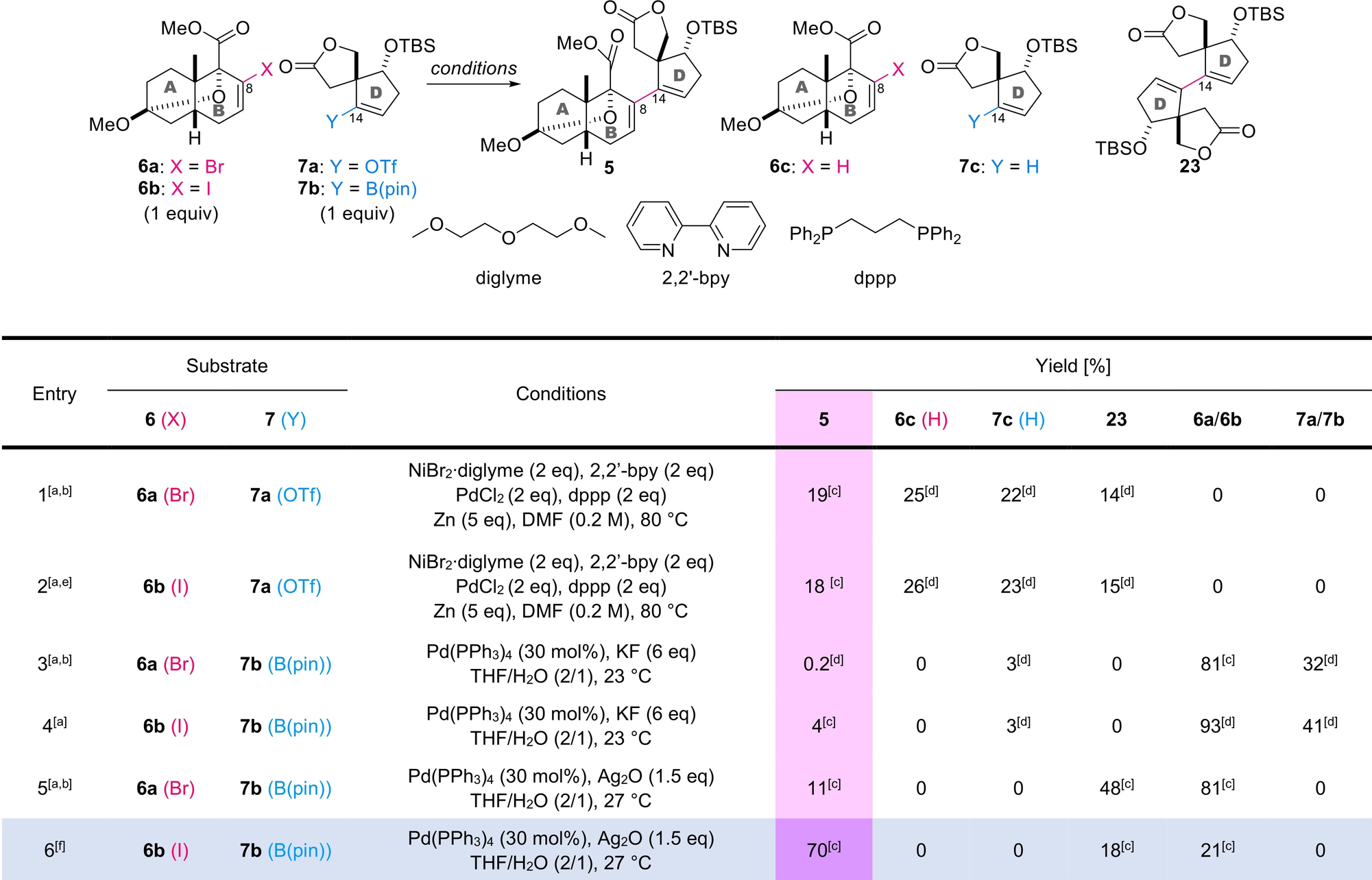
- [a] 20–40 mg scale. [b] 6 a (C11−OMe) was used as a mixture with 6 a′ (C11−OEt) [6 a (C11−OMe):6 a′ (C11−OEt)=8 : 1]. [c] Yield of the isolated product. [d] Yield calculated from 1H NMR data. [e] 6 b (C11−OMe) was used as a mixture with 6 b′ [6 b (C11−OMe):6 b′ (C11−OEt)=8 : 1]. [f] 480 mg scale. 2,2′-bpy=2,2′-bipyridine, dppp=1,3-bis(diphenylphosphino)propane.
According to the proposed mechanism of the Weix coupling (Scheme 3a),26 the Ni0 reagent reacts with vinyl halide 6 a/b to produce 24 and the Pd0 reagent oxidatively adds to vinyl triflate 7 a to afford 26. Transmetalation converts NiII species 24 into ZnII species 25, which reacts with PdII species 26 to furnish adduct 5 through reductive elimination of Pd0 from 27. Thus, the byproducts 6 c and 7 c are likely to originate from the direct protonation of 24/25 and 26, respectively, on the work-up. Alternatively, the formation of dimer 23 is attributable to undesired generation of the vinyl zincate of the D-ring and its transmetalation with 26, followed by reductive elimination. Hence, the unproductive side reactions indicate the innately slow process from 25 and 26 to the key intermediate 27 at the sterically shielded C8 and C14 positions.
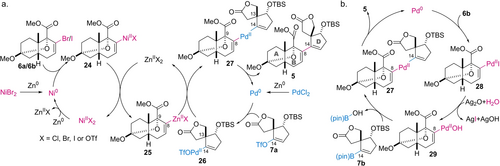
Mechanistic rationale of a) the Pd/Ni-mediated Weix coupling and b) Pd/Ag-promoted Suzuki–Miyaura coupling.
The above negative results led us to adopt the Suzuki–Miyaura coupling reaction using pinacol boronate 7 b (Table 1).27 As the presence of the base-sensitive lactone of 7 b prevented us from employing commonly used strong bases (e.g., KOH, KOEt), we first utilized standard and less basic KF28 as a promoter of the coupling. Only a trace amount of 5 (0.2 %) was obtained from the coupling using bromide 6 a, boronate 7 b, Pd(PPh3)4, and KF in aqueous THF at 23 °C (entry 3). Starting compounds 6 a and 7 b were mainly recovered. To accelerate the oxidative addition of Pd0, iodide 6 b was used instead of bromide 6 a under otherwise same conditions, but the yield of 5 improved to only 4 % (entry 4). The data in entries 3 and 4 suggested that the slow transmetalation step impeded the promotion of the catalytic cycle of the reaction. Thus, Ag2O was examined as the base, because of its potential ability to accelerate this critical step.29 As evident from the comparison between entries 3/4 and 5/6, this promoter resulted in a dramatic increase in the yield of the desired 5 to 11 % from bromide 6 a and 70 % from iodide 6 b. In the optimized conditions (entry 6), AB-ring 6 b (1 equiv) and D-ring 7 b (1 equiv) were treated with Pd(PPh3)4 (30 mol%) and Ag2O (1.5 equiv) in aqueous THF at 27 °C to produce 5 in 70 % yield with the isolation of AB-ring 6 b (21 %) and D-ring dimer 23 (18 %). Remarkably, the newly established conditions realized the highly challenging C8−C14 bond formation in almost quantitative yield (89 % based on recovered 6 b) under mild conditions at ambient temperature.
Although Ag2O has not been commonly employed as the base for Suzuki–Miyaura coupling reactions in natural product synthesis, its superiority to KF was validated by the data in entries 3–6 (Table 1). A scenario of the Pd/Ag-promoted catalytic reaction is proposed based on experimental and theoretical precedents (Scheme 3b).30, 31 First, the oxidative addition of Pd0 to iodide 6 b generates 28. The transformation of 28 to hydroxy-palladium complex 29 is greatly accelerated by Ag2O through precipitating the insoluble salt, AgI. The formation of 29 should override the steric congestion and drive the catalytic cycle forward. Hence, Lewis-basic 29 coordinates to the three-coordinated Lewis-acidic boron atom of boronic acid ester 7 b. This coordination significantly facilitates the transmetalation to obtain the key intermediate 27. Finally, reductive elimination of Pd0 from 27 provides the desired adduct 5, thereby closing the catalytic cycle.
The thus-obtained adduct 5 was equipped with two carbonyl groups for the Dieckmann condensation reaction, allowing for the two-step construction of steroidal structure 4 (Scheme 4). Treatment of 5 with potassium tert-butoxide in THF at 0 °C resulted in C-ring cyclization within 10 min through C12−H deprotonation, nucleophilic attack, and methoxide elimination, leading to 30. In situ addition of n-Bu4NF detached the TBS group of 30 to afford the secondary alcohol of 31 in 79 % yield as a single C12-diastereomer.32 The extra C12′-carbonyl group was then removed by the expulsion of CO2 to yield steroidal skeleton 4. Specifically, hydrolysis of the lactone of 31 with LiOH in aqueous THF, acidification by HCl, and thermal decarboxylation at 135 °C in o-dichlorobenzene furnished 4. Noteworthily, the spirally fused lactone implemented in the intermediates plays three roles: it avoids the use of the protective groups for C18-hydroxy and C12′-carboxy groups, minimizes the steric hindrance, and lowers the pKa value of the C12−H.33 These enabling functions simplified the scheme en route to 4 and permitted the crucial Suzuki–Miyaura and Dieckmann reactions under mild conditions without affecting potentially reactive functional groups.
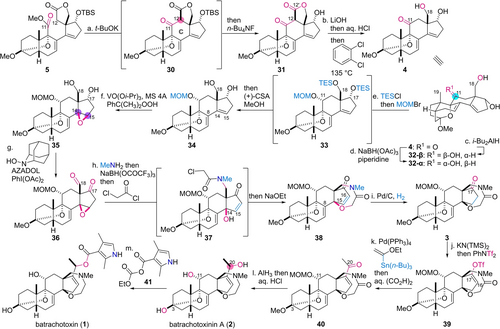
Total synthesis of batrachotoxinin A (2) and batrachotoxin (1). Reagents and conditions: a) t-BuOK, THF, 0 °C, then n-Bu4NF, 23 °C, 79 %; b) LiOH⋅H2O, THF/H2O (2/1), 22 °C, then aq. HCl, 0 °C, then o-dichlorobenzene, 135 °C, 77 %; c) i-Bu2AlH, CH2Cl2/Et2O (3/1), −78 °C, 36 %, (56 % based on recovered 4); d) NaBH(OAc)3, piperidine, MeCN/DMF (2/1), 22 °C, 32-α: 73 %, 32-β: 18 % (two cycles); e) triethylsilyl chloride (TESCl), i-Pr2NEt, N,N-dimethyl-4-aminopyridine (DMAP), (CH2Cl)2, 21 °C, then bromomethyl methyl ether (MOMBr), i-Pr2NEt, 40 °C, then (+)-CSA, MeOH, 0 °C, 89 %; f) VO(Oi-Pr)3 (25 mol%), PhC(CH3)2OOH, molecular sieves 4A (MS 4A), CH2Cl2, 0 °C, 70 %; g) AZADOL (15 mol%), PhI(OAc)2, CH2Cl2, 27 °C; h) MeNH2, CH2Cl2, 24 °C, then NaBH(OCOCF3)3, CH2Cl2, −78 °C, then ClCOCH2Cl, 2,6-lutidine, −78 °C to 0 °C, then NaOEt, EtOH, 0 °C; i) Pd/C, H2, EtOAc, 24 °C, 38 % (three steps from 35); j) KN(TMS)2, THF, −78 °C, then PhNTf2, −78 °C, 86 %; k) Pd(PPh3)4 (15 mol%), tributyl(1-ethoxyvinyl)stannane, LiCl, CuCl, THF, 60 °C, then aq. (CO2H)2, 0 °C, 77 %; l) AlH3, THF, −78 °C to 0 °C, then aq. HCl, 25 °C, 2: 53 %, epi-2: 6.9 %; m) 41, Et3N, benzene, 35 °C, 53 % (two steps from 40).
Having built steroidal structure 4, the oxidation level of the C-ring was adjusted in three steps (Scheme 4). First, the α-oriented secondary C11-hydroxy group was constructed by exploiting the primary C18-alcohol on the β-face as a directing group. A typical hydride source such as i-Bu2AlH only approached from the opposite face of the β-oriented C18- and C19-substituents of 4 to generate the undesired β-oriented alcohol 32-β. To invert this innate stereoselectivity, a reagent combination of NaBH(OAc)3 and piperidine was utilized to transfer a hydride from the temporarily formed C18-hydroborate, thereby converting the C11-ketone of 4 into the requisite α-oriented hydroxy group of 32-α (dr at C11=4.1 : 1).34, 35 The resultant secondary C11-hydroxy group of 32-α was protected with the MOM group of 34 in one pot. Namely, after TES protection of the more accessible C17- and C18-hydroxy groups of triol 32-α with TESCl/i-Pr2NEt, sequential treatment with MOMBr and (+)-CSA/MeOH led to MOM protection and TES removal, respectively, affording diol 34. The next installation of the β-oriented C14-oxygen functionality to diene 34 was realized again using the proximal C18-hydroxy group as the chemical handle. Treatment of 34 with a catalytic amount of VO(Oi-Pr)3 in the presence of cumene hydroperoxide and MS 4A furnished β-epoxide 35 as a major product.36 In this reaction, the vanadium-oxidant complex would be site-selectively formed at the less hindered C18-hydroxy group over the C17-hydroxy group. The C18-vanadate would then differentiate the C8- and C14-double bonds based on the distance as well as the two C14-olefin faces based on the orientation to achieve the desired regio- and stereoselectivity.
Next, the oxidized lactam was fused with the CD-ring as the oxazepane surrogate by utilizing the oxygen functional groups on the D-ring. The C17- and C18-hydroxy groups of 35 were oxidized together to the corresponding carbonyl groups of 36 by catalytic AZADOL in the presence of PhI(OAc)2. The one-pot procedure was then devised for transforming 36 to 38 to avoid the isolation of unstable intermediates. Treatment of 36 with MeNH2 in CH2Cl2 simultaneously induced the formation of the C18-imine and the tertiary C14-hydroxy group by nucleophilic addition and the E1cB reaction of the epoxide, respectively. Sequential in situ addition of NaBH(OCOCF3)3, chloroacetyl chloride/2,6-lutidine, and NaOEt/EtOH, resulted in reductive C18-amination, α-chloro acetylation of the amine, and intramolecular C14-etherification of 37, giving rise to seven-membered lactam 38. The ensuing regioselective hydrogenation of the C15-disubstituted olefin of 38 over the C8-trisubstituted olefin by Pd/C catalysis provided ketone 3. Hence, the entire ring system was assembled at this stage.
The last four steps, including the chain extension on the D-ring, completed the total synthesis of 1. The five-membered ketone of 3 was chemoselectively converted to the vinyl triflate of 39 using KN(TMS)2 and PhNTf2 in THF with the intact seven-membered lactam. Stille coupling37 between 39 and tributyl(ethoxyvinyl)tin occurred at 60 °C by the action of a catalytic amount of Pd(PPh3)4 and a stoichiometric amount of LiCl and CuCl. The generated vinyl ether was hydrolyzed using aqueous oxalic acid in one pot, leading to methyl ketone 40. Then, application of AlH338 realized the reduction of the lactam to oxazepane as well as stereoselective conversion of the C20-ketone into the C20-hydroxy group (dr at C20=7 : 1). Acidic work-up with aqueous HCl in turn detached the C3O−Me and C11O−MOM groups. The following HPLC purification produced batrachotoxinin A (2) (53 % yield) and its C20-epimer epi-2 (6.9 % yield). The total synthesis of 1 was more conveniently accomplished using the C20-diastereomeric mixture of 2 and epi-2 without HPLC purification. Thus, 2,4-dimethyl-1H-pyrrole-3-carboxylic acid was conjugated with the C20-alcohol group of 2 and epi-2 by employing anhydride 41, delivering (−)-batrachotoxin (1) in 53 % yield from 40 after flash column chromatography.39 Comparison of the analytical data of the obtained 1 (1H and 13C NMR) with those of the reported 1 fully corroborated the structural integrity of our synthetic 1.
Conclusion
In summary, we synthesized the densely functionalized 6/6/6/5-membered ABCD-ring structure of batrachotoxin (1) in 22 steps from (+)-Wieland-Miescher ketone (8) (0.71 % yield). The total synthesis consisted of three stages. In the first stage, we efficiently prepared AB-ring 6 b by taking advantage of the cis-fused shape of the intermediates, and D-ring 7 b by employing desymmetrizing asymmetric hydrogenation. Compounds 6 b and 7 b were equipped with five of the eight stereocenters of 1 as well as all the functionalities of the subsequent C-ring formation. These structural features of the two fragments expedited the entire scheme to 1. In the second stage, we devised new methods for assembling the fragment and cyclizing the C-ring to build the steroidal skeleton 4. The Pd/Ag-promoted Suzuki–Miyaura coupling reaction between 6 b and 7 b effectively forged the highly hindered bond of 5 at the C8 and C14 positions. The Dieckmann condensation successfully cyclized the C-ring, and the following decarboxylation expelled the extra carbon to provide 4. In the third stage, the judiciously optimized regio-, chemo-, and stereoselective functionalizations on the CD-ring converted 4 into target 1. The C18-hydroxy group was exploited to stereoselectively install the C11- and C14-oxygen-substituted stereocenters. Formation of the oxazepane ring using chloroacetyl chloride, introduction of the D-ring side chain by the Stille coupling conditions, and reductive installation of the C20-hydroxy group gave rise to batrachotoxinin A (2). The biologically significant 2,4-dimethyl-1H-pyrrole-3-carboxy group was then introduced to complete the total synthesis of 1. Overall, the present convergent strategy and the developed highly selective transformations were demonstrated to be applicable to complex steroidal intermediates, and should thus provide new valuable information for the development of routes to numerous oxygenated steroids with diverse pharmacologically important activities. Furthermore, the powerful Pd/Ag-promoted Suzuki–Miyaura coupling reactions should serve as an enabling technology for forging hindered bonds within the structures of many densely functionalized natural products beyond this study.
Supporting Information
The authors have cited additional references within the Supporting Information.40-42
Acknowledgments
This research was financially supported by Grants-in-Aid for Scientific Research (S) (JP22H04970) to M.I. and for Early Career Scientists (JP21K14622) to K.H. from JSPS. A fellowship from MEXT (WISE program) to Y.W. and from JSPS (JP17J09521) to H.M. is gratefully acknowledged. We thank Dr. K. Sakata and Dr. D. Kamakura for conducting the preliminary experiments for this study.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




